Melchiorre's route for the synthesis of chiral molecules highlighted in Synfacts
A paper published by Prof. Melchiorre’s group in Nature has been highlighted in Synfacts, vol 12, issue 7 (739). Synfacts selects the most significant results on synthetic organic chemistry that appear in the literature considered as the future trends in synthetic chemistry.
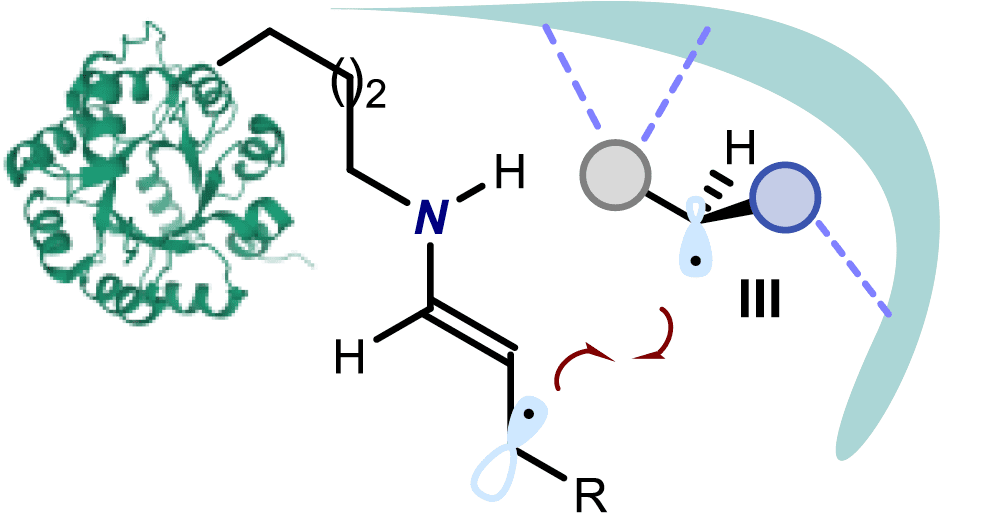
In this paper the authors show how the combination of photoredox and asymmetric organic catalysis enables enantioselective radical conjugate additions to β,β-disubstituted cyclic enones to obtain quaternary carbon stereocentres with high fidelity. Critical to the success is the design of a chiral organic catalyst, containing a redox-active carbazole moiety, that drives the formation of iminium ions and the stereoselective trapping of photochemically generated carbon-centred radicals by means of an electron-relay mechanism. The researchers demonstrate the generality of this organocatalytic radical-trapping strategy with two sets of open-shell intermediates, formed through unrelated light-triggered pathways from readily available substrates and photoredox catalysts—this method represents the application of iminium ion activation (a successful catalytic strategy for enantioselective polar chemistry) within the realm of radical reactivity.
Ref:
Asymmetric catalytic formation of quaternary carbons by iminium ion trapping of radicals
J. J. Murphy, D. Bastida, S. Paria, M. Fagnoni, P. Melchiorre
Nature, 532, 218-222
Related news

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements







 22-11-2024
22-11-2024