Melchiorre's paper: Significant contribution to Angewandte's 2011 Impact Factor
The generation of all-carbon quaternary stereocenters and the construction of highly strained polycyclic structures is one of the greatest challenges for organic chemists. Both are structural features of many natural products and medicinal compounds.
The spirocyclic oxindole core is an example of these challenging structures.
In this paper, Paolo Melchiorre and his team develop a direct one-step synthesis of spirocyclic oxindoles by asymmetric organocascade catalysis. They use two different organocatalysts that activate carbonyl compounds, such as ketones and aldehydes, with extraordinary levels of stereocontrol.
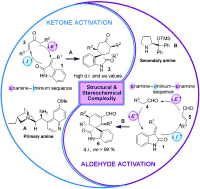
If you want to find out about those catalysts read the article here and to see the list of articles that, together with this one, have contributed the most to Angewandte’s 2011 impact factor go here.
Giorgio Bencivenni, Li-Yuan Wu, Andrea Mazzanti, Berardino Giannichi, Fabio Pesciaioli, Mao-Ping Song, Giuseppe Bartoli, Paolo Melchiorre
Angew. Chem. Int. Ed. 2009, vol. 48, pp. 7200–7203
Related news

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements






 11-12-2024
11-12-2024