DOSY: the importance of getting the timing right
24th March 2020 – Researchers from the Ballester group, in collaboration with the Rebek group from The Scripps Research Institute and Shanghai University, and the Cohen group from Tel Aviv University, and the help provided by the members of the ICIQ NMR unit, have used diffusion-ordered NMR spectroscopy (DOSY), to characterise water-soluble bis-formamide caviplexes and dive into their kinetic stabilities and exchange dynamics. Luis Escobar, former PhD student of the Ballester group and first author of the paper, published in Chemistry – A European Journal, shares the story behind it:
What have you done?
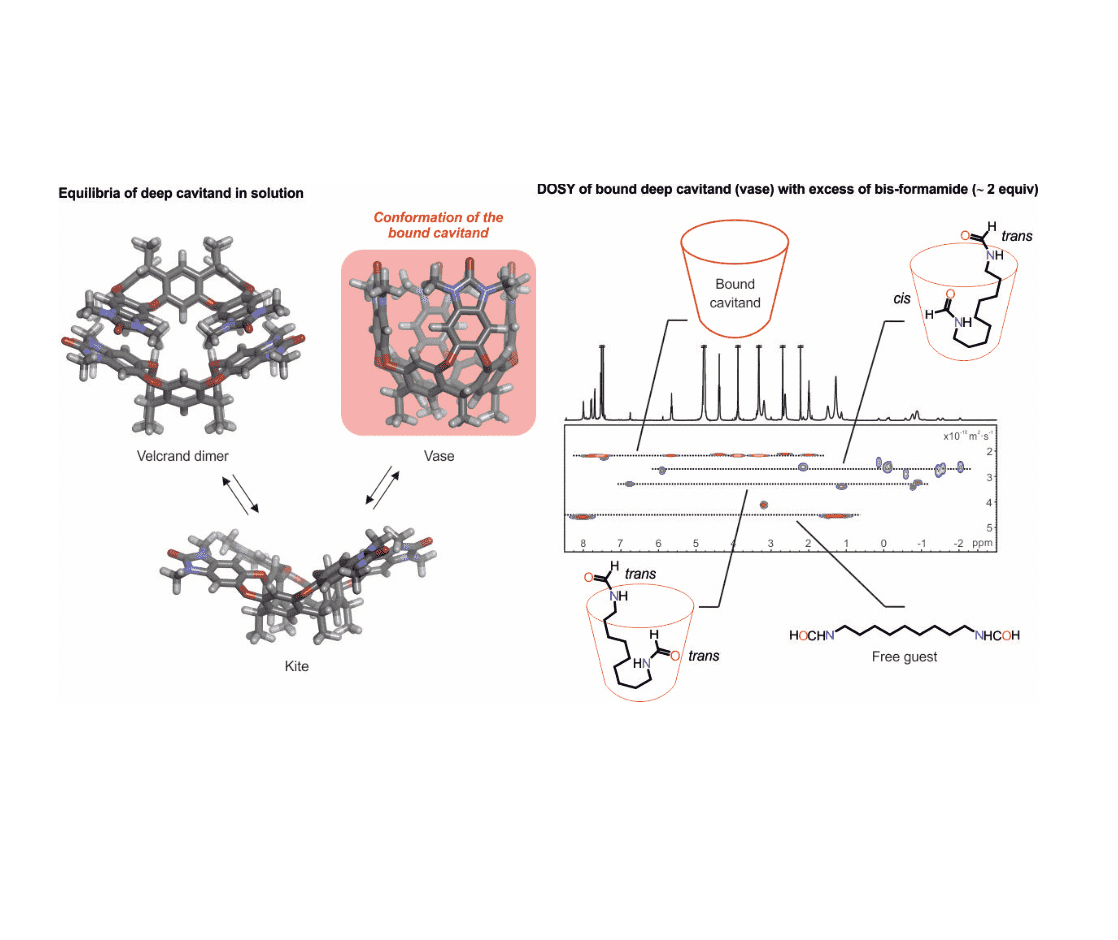
Diffusion-ordered NMR spectroscopy (DOSY) is a well-established method that reports translational diffusion constant values. Together with our collaborators we published a paper in PNAS about the relative hydrophilicities of trans and cis formamides, in which we used direct competition for their binding to a water-soluble deep cavitand. During these studies, we also applied DOSY to characterise the host-guest complexes and realised our results weren’t aligned with the main school of thought. We believed that this was because there are scarce examples in the literature of using DOSY for the kinetic characterisation of supramolecular complexes. So, we decided to investigate the kinetic stabilities and the exchange processes involved in the complexes of long-chain bis-formamide guests and the cavitand receptor in water using DOSY.
When a bis-formamide is added in excess to the cavitand, two isomeric 1:1 host-guest inclusion complexes (caviplexes) are formed: trans,trans– and trans,cis-bis-formamide caviplexes. If the host and guest form a complex, both components diffuse as a single species and you’ll expect analogous diffusion constant values for the bound host and the bound guest, which are different from that of the free guest. However, if exchange processes are happening at the diffusion timescale, then the result is an apparent diffusion constant for the bound guest that does not coincide with neither the bound host nor the free guest. What we report in this paper, is that the observation of exchange processes going on at DOSY depends on the diffusion time used. When we performed DOSY experiments with different diffusion times we realised that with short diffusion times the exchange process is slow and thus, the diffusion constants of the bound host and the bound guest coincide, but at longer diffusion times, the guest separates in the diffusion scale from the bound host towards the diffusion constant of the free guest (intermediate exchange). What we want to stress, is that researchers need to be careful when analysing DOSY experiments because one can reach wrong conclusions: if after a DOSY experiment one is not certain about the results, we can change the diffusion time to understand what’s happening. When doing a DOSY experiment with a diffusion time relatively long for the bis-formamide caviplexes, one can observe that one of the isomers (trans,cis) has a diffusion constant value more similar to the bound receptor than the other one (trans,trans), which shows the kinetic stability of the host-guest complexes. This is how we discovered that the trans,cis-isomer is more kinetically stable, and more thermodynamically stable, than the trans,trans-counterpart.
Why is this important?
We believe it’s important because DOSY is a general technique, applicable to many areas of research from organometallic or organic chemistry to simple biological systems. We think that one of the issues is that we often use DOSY a bit blindly, so in this paper we try to explain what can happen when you do a DOSY and how to analyse its results in detail. When doing NMR experiments, we’re aware of the 1H NMR chemical shift timescale, or that EXSY uses another timescale. What we learnt is that in DOSY there’s another temporal scale – and we need to take it into consideration, especially when dealing with dynamic systems.
Reference paper:
Related news

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements







 20-02-2025
20-02-2025 



















