Chiral molecule synthesis: New light-activated enzyme enables precise stereocontrol
12th September 2024 -
ICIQ researchers publish in Nature a new strategy to enantiocontrolled chemistry using light and enzymes
Researchers from the Institut Català d’Investigació Química (ICIQ-CERCA) have developed a novel strategy for synthesizing chiral molecules with precise enantiocontrol, a new way to control the production of a specific enantiomer. This strategy offers a powerful new tool for the synthesis of chiral molecules with potential applications in pharmaceuticals, advanced materials production and other fields where stereochemistry is essential.
Our study introduces a novel approach to synthesising chiral molecules by combining photochemistry, biocatalysis, and organocatalysis. These three powerful strategies, when integrated, enable the creation of sustainable methods for producing complex chiral compounds that are challenging to access using conventional techniques.
Prof. Paolo Melchiorre, the principal investigator of this work
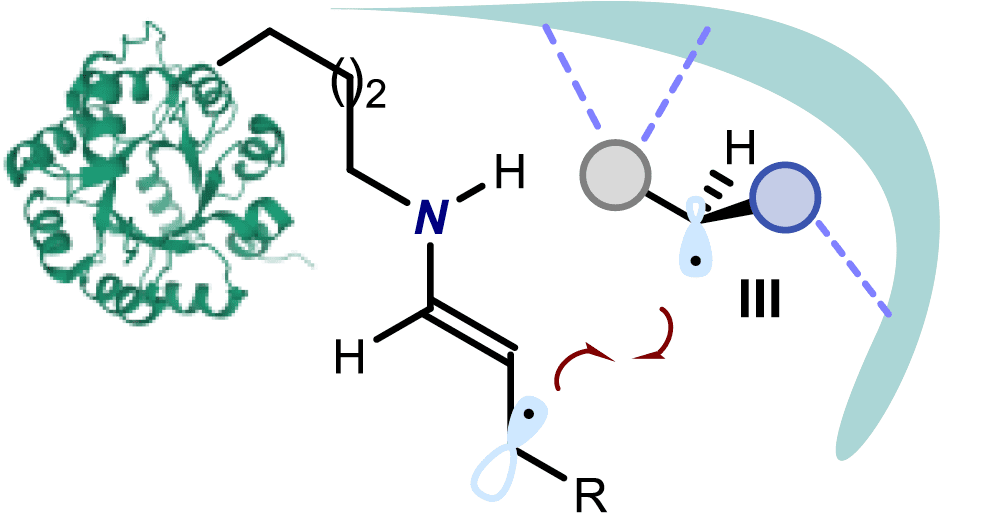
The study has been published in the prestigious journal Nature and describes the development of a non-natural photodecarboxylase that forms radicals upon activation of chiral carboxylic acids to then enable a stereospecific C-C bond forming process. This photoenzyme uses a unique mechanism of photoactivation to mediate a transformation which is beyond the reach of small-molecule catalysts.
Inspired by Nature
Recent studies have demonstrated that biocatalysts (molecules of biological origin used to speed up chemical reactions) offer effective environments for enantioselective catalysis. In particular, photoenzymes, which use photonic energy to carry out chemical transformations, have emerged as interesting tools for enantiocontrolled synthesis.
Prof. Melchiorre’s group have engineered an artificial enzyme capable of harnessing visible light to activate specific chemical intermediates. This work explores a novel photoenzymatic strategy that diverges from the traditional approach. Instead, the researchers focused on the direct excitation of protein-bound catalytic intermediates.
Through directed evolution, we enhanced the enzyme’s catalytic abilities, enabling it to carry out reactions that are not possible in nature.
Prof. Melchiorre
The researchers anticipate that this photoenzymatic strategy is versatile enough to enable the advancement of other unconventional stereocontrolled radical functionalisation processes.
Celebrating ICIQ contribution to this journey
In September 2009 Prof. Melchiorre joined ICIQ, but in 2022 he moved back to his Alma Mater, the University of Bologna. However, part of his group is performing research at ICIQ, as it is demonstrated with this work, conducted entirely by Melchiorre’s group at ICIQ. Today, the group still includes two PhD students, though it is nearing its end. With this work the ICIQ Melchiorre’s group will have a happy ending.
I am particularly proud of this study for several reasons. First and foremost, it represents the final project my research team developed at ICIQ, a place where I spent a significant part of my career and that provided me with invaluable support to advance my work. ICIQ has been instrumental in allowing me to evolve scientifically, from organocatalysis to photochemistry, and now combining both with biocatalysis—a journey I could have undertaken in only a few places, and ICIQ is certainly one of them.
Prof. Melchiorre
I am also pleased to see how my young coworkers succeeded in bringing together diverse expertise, from protein engineering to synthetic chemistry, while demonstrating an exceptional team spirit. Their ability to collaborate, combine different skills, and work toward a shared goal was essential for the success of such a multidisciplinary project. I am grateful for their dedication, talent, and the collaborative environment we fostered together.
Reference article
Stereospecific radical coupling with a non-natural photodecarboxylase
Tseliou, V.; Kqiku, L.; Berger, M.; Schiel, F.; Zhou, H.; Poelarends, G. J.; Melchiorre, P.
Nature 2024, DOI: 10.1038/s41586-024-08004-9.
Related news

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements






 27-03-2025
27-03-2025 















