ChemistryViews highlights ICIQ Research
Vladimir Grushin and co-workers published a new method for the trifluoromethylation of aryl boronic acids in Angew. Chem. Int. Ed. last June.
Now, ChemistryViews highlights this ‘Low Cost, Simple, Efficient, Safe Trifluoromethylation’ in its latest issue.
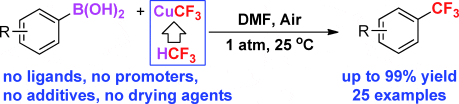
In this paper, Petr Novák, Anton Lishchynskyi and Vladimir Grushin demonstrate, for the first time, that aryl boronic acids undergo smooth and selective trifluoromethylation with low-cost fluoroform-derived CuCF3 in DMF in non-dried air. The reaction occurs at room temperature, exhibits unprecedented functional-group tolerance and affords trifluoromethylated aromatic compounds in up to 99 % yield.
This is a simple, inexpensive and safe method to obtain libraries of aromatic compounds bearing a CF3 group on the ring for the synthesis of biologically active compounds (pharmaceuticals and agrochemicals) as well as specialty materials.
Related news

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements






 27-05-2022
27-05-2022 


















