Capturing elusive cobalt intermediates
Cobalt catalysts are becoming a popular alternative to more expensive metals like rhodium and palladium for the functionalisation of C–H bonds. Recently, Cp*CoIII complexes demonstrated being effective in the formation of carbon-carbon and carbon-halogen bonds. Nevertheless, the lack of understanding of the mechanistic insights of these systems hinders the development of new reactivities.
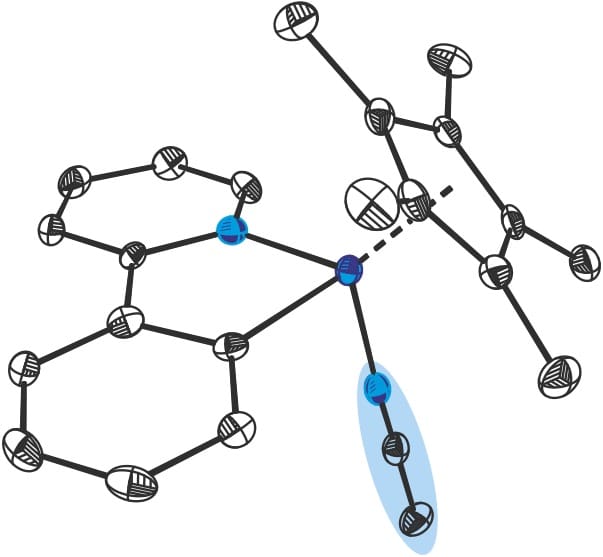
Now, a group of researchers at ICIQ led by Dr. Mónica H. Pérez-Temprano, successfully identified and characterised an elusive cobaltacycle intermediate that could help better understand how Cp*CoIII complexes catalyse C–H functionalisation reactions. In particular, the ‘captured’ intermediates shine light on a cobalt-catalysed oxidative alkyne annulation.
Chemists synthesized a new Cp*CoIII pre-catalyst featuring an acetonitrile molecule in its structure. When added to the reaction mixture, this molecule leads to the formation of a long-lived intermediate that appears in the NMR spectrum. Pérez-Temprano’s team went even further and managed to fully characterise it using X-ray crystallography. They propose that acetonitrile may play a key role in stabilising otherwise highly reactive cobalt intermediates.
ICIQ chemists also found reaction conditions that allow incredibly low catalyst loadings of down to 1 mol%. To date, the methodology developed by Pérez-Temprano and co-workers represents the lowest catalyst loading in this type of cobalt-catalysed annulation reactions.
Capturing elusive cobaltacycle intermediates: a real-time snapshot of the Cp*CoIII-catalyzed oxidative alkyne annulation.
Sanjosé-Orduna, D. Gallego, A. Garcia-Roca, E. Martin, J. Benet-Buchholz, M.H. Pérez Temprano.
Angew. Chem. Int. Ed., 2017, DOI: 10.1002/anie.201704744.
Related news

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements






 30-10-2024
30-10-2024 

















