19/11/2015
19/11/2015
 15:30
15:30
 ICIQ Auditorium
ICIQ Auditorium
- Lecturer: Prof. Varinder K. Aggarwal
- University: University of Bristol (United Kingdom)
Assembly Line Synthesis
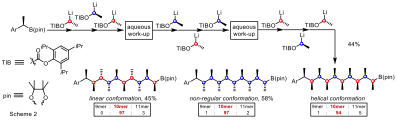
In the biosynthesis of polyketides, Nature takes a simple building block and through a series of iterative enzymatic reactions [polyketide synthases (PKS)] manufactures a vast array of secondary metabolites, many of which display high chemical complexity and biological activity. We propose to try to emulate Nature’s remarkable structural and functional diversity in assembly of polyketides through a related strategy. In particular we have taken simple boronic esters and carried out iterative homologations using primary and secondary lithiated carbamates, enabling us to grow carbon chains with control over both relative and absolute stereochemistry. Applications of this strategy to natural and non-natural products will be demonstrated.
The secondary and tertiary boronic esters formed at the end of the homologation sequence can be converted into a range of functional groups. I will show a new method for the stereospecific coupling of these hindered chiral boronic esters with aryl halides.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements