07/11/2022
07/11/2022
 12:00 h
12:00 h
- Lecturer: Prof. Masahiro Murakami
- University: Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University (Japan)
- Sponsored by:

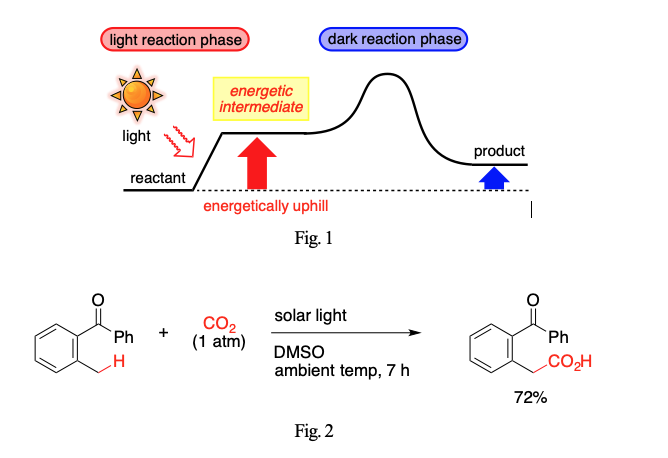
Utilization of Energy of Photons for Organic Synthesis
Whereas thermal reactions give rise to products that are thermodynamically more stable than reactants, photoreactions are able to furnish products that are more energetic than reactants. Such endergonic processes offer a unique way to transform compounds of inert nature such as CO2. In this lecture are presented synthetic reactions driven by the energy of photons. Chemical bonds and/or entities that have been conventionally considered to be stable and inert are activated for a reaction with the aid of photons. The fundamental scheme underlying our strategy consists of two phases, one corresponding to “light reaction” of photosynthesis, and the other corresponding to “dark reaction” (Fig. 1). The first light reaction phase is a photo-induced endergonic process. A photon donates energy to a reactant to produce a more energetic intermediate. The second dark reaction phase is a thermal process converting the energetic intermediate to a desired product. The energy gained in the first light reaction phase provides a driving force required for the second dark reaction phase. The net transformation can be energetically uphill, which is impossible with thermo-driven transformations in principle. MM will discuss a few examples that fit in the scheme of two-phase mechanism, including the one shown in Fig. 2.1
[1] Y. Masuda, N. Ishida, M. Murakami, J. Am. Chem. Soc. 2015, 137, 14063.

Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements