Ring-Opening of Cyclic Carbonates: From Fine Chemicals to CO2-based Polymers
Cyclic carbonates have unique reactivity comprising both nucleophilic and electrophilic sites. This dual behavior allows expanding the portfolio of functional intermediates and monomers derived from a CO2-derived heterocycle. So far, the ring-opening chemistry of these heterocycles has been mostly limited to carbonate-attack approaches, which are useful in the context of fine-chemical (linear carbamates) and polymer chemistry (polyurethanes) applications. Much less explored are nucleophilic attacks on positions that are referred to as “methylene” and “exo-cyclic” in the cyclic carbonate precursor, and new approaches that can unleash effective and selective protocols for such pathways will help to increase the applicability of these CO2 based heterocycles in synthetic chemistry. Several new types of ring-opening processes have been studied in this thesis and details of these advancements are described below.
In the first project (Chapter 2), an extraordinary ring-opening process of cyclic carbonates bearing vinyl groups is studied. A unique sequence of decarboxylation, CH3CN extrusion and Michael addition is noted when vinyl cyclic carbonates are treated with cyanide at elevated temperatures. β-cyano ketones are produced under these conditions in moderate to high yields. The scope of this process is further discussed and a series of mechanistic control experiments (including labeling studies) is presented that allow to propose a viable formation mechanism of the β-cyano ketone products.

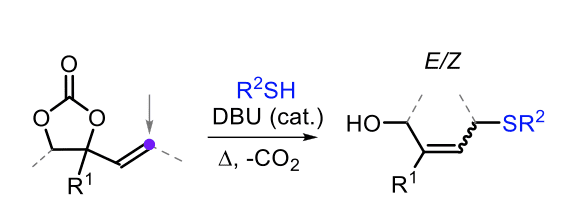
In the second project (Chapter 3), the nucleophilic attack of alkyl- and aryl-thiols onto vinyl cyclic carbonates is examined using DBU as an organocatalyst. This process results in the formation of a series of allylic thioether compounds in moderate to good isolated yields. The process can be seen as a formal allylic substitution reaction that does not require a transition metal, and can be carried out under relatively mild conditions. Several control experiments are presented to validate the mechanistic hypothesis that the base is required to activate the thiol pro-nucleophile towards the attack on the C-terminus of the exo-cyclic double bond.
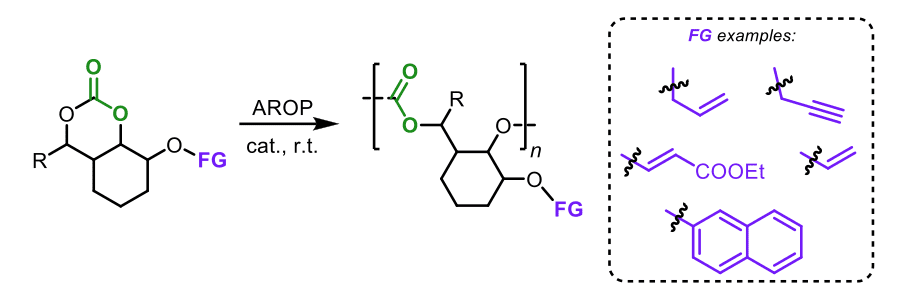
In the third project (Chapter 4), a diverse series of functional bicyclic monomers is investigated for their propensity for ROP in the presence of TBD as catalyst and benzyl alcohol (BnOH) as initiator. A direct route to densely substituted macromolecular aliphatic carbonates is developed including bifunctional monomers capable of initiating two distinct ring-opening polymerization processes offering versatile options for polymerization and crosslinking strategies. The controlled degradation of a selected polycarbonate is also examined using TBD as catalyst leading to a new type of bicyclic oxetane.
In conclusion, the results presented in this thesis show that (functionalized) cyclic carbonates are versatile synthons for the preparation of a wide variety of new and functional heterocyclic products, and aliphatic polycarbonates. These approaches significantly expand the repertoire of the applications that have been developed so far in the field of non-reductive conversions of CO2 and their use as intermediates in new synthetic transformations.

If you would like to follow the ceremony on ZOOM, please register here
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements
 24/10/2023
24/10/2023
 11:00 h
11:00 h
 ICIQ Library
ICIQ Library




















