Regiospecific N-Arylation of Nitrogen Nucleophiles under Mild and Metal-Free Conditions
Aniline and amide derivatives are ubiquitous in Nature, in medicinal applications and material science. Consequently, the research focus on development of efficient synthetic methodology for N- arylation of nitrogen nucleophiles remains intense. While transition metal-catalyzed cross couplings have been successful with a wide variety of nitrogen nucleophiles, the drawbacks associated with transition metal catalysis, including toxicity, cost, need for substrate-dependent designer ligands, and risk of product contamination has led to an increased focus on development of metal-free methodology for C-N bond formation.
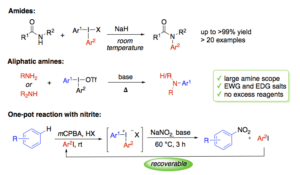
Diaryliodonium salts are environmentally benign, reactive and selective electrophilic arylation reagents. We have developed several one-pot syntheses of diaryliodonium salts, making these reagents easily available. We have also demonstrated the efficiency of iodonium salts in arylation of various heteroatom and carbon nucleophiles under mild and metal-free conditions. Arylation of certain nitrogen nucleophiles is well established, but the high pKa of many N-nucleophiles make them difficult to arylate without byproduct formation. Reactions with aliphatic amines are difficult, and limited to arylation of cyclic, secondary amines with electron-withdrawing iodonium salts.
In this lecture, our results on the N-arylation of amides, aliphatic amines and nitrite will be presented, together with our mechanistic understanding of how these reactions proceed. The first sequential one-pot reaction, where diaryliodonium reagents are formed from iodine(I), and subsequently trapped by in situ addition of N-centered nucleophiles, will also be described.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements
 12/12/2017
12/12/2017
 09:30
09:30