 05/03/2015
05/03/2015
 12:00 h
12:00 h
 ICIQ Auditorium
ICIQ Auditorium
- Lecturer: Prof. Masanari Kimura
- University: Nagasaki University (Japan)
- Sponsored by:

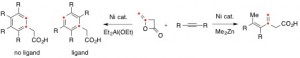
Regio- and Stereoselective C-C Bond Formation via Oxanickelacycles
Diketene is often used as acetoacetylating reagent for versatile nucleophiles, such as alcohols, amines, thiols and carbanions in organic synthesis. In the presence of Ni-catalyst, diketene reacts with organometallic compounds, such as Grignard reagents and organozinc reagents, to undergo the vinyl-oxygen bond cleavage reactions to form 3-substituted 3-butenoic acids. 3-Butenoic acid skeleton serves as a synthon for the preparation of isoprenoid acid and as a monomer for polymerization, and are successfully utilized for the synthesis of physiologically active molecules. Herein, we would like to report the selective formations of unsaturated carboxylic acids and phenylacetic acids from diketene with alkynes promoted by Ni-catalytic systems in a single manipulation.[1] In the presence of Ni catalyst, three components of diketene, alkyne, and Me2Zn combine smoothly to provide 3-methylene-4-hexenoic acids in excellent yields. Under similar catalytic system, Et2Al(OEt) promotes [2+2+2] cycloaddition reactions with diketene and two equivalents of alkyne to give rise to the substituted phenylacetic acids. More interestingly, in the presence of Et2Al(OEt) and triphenylphosphine ligand, symmetrical phenylacetic acids are constructed via a formal [2+2+1+1] cycloaddition reaction with diketene and two equivalents of alkynes accompanying the cleavage reactions of methylene C=C double bond of diketene.
References
[1] Mori, T.; Akioka, Y.; Kawahara, H.; Ninokata, R.; Onodera, G.; Masanari K, Angew. Chem. Int. Ed. 2014, 53, 10434.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements