
 19/06/2024
19/06/2024
 11:00
11:00
- Lecturer: Prof. Linda Shimizu
- University: University of South Carolina (USA)
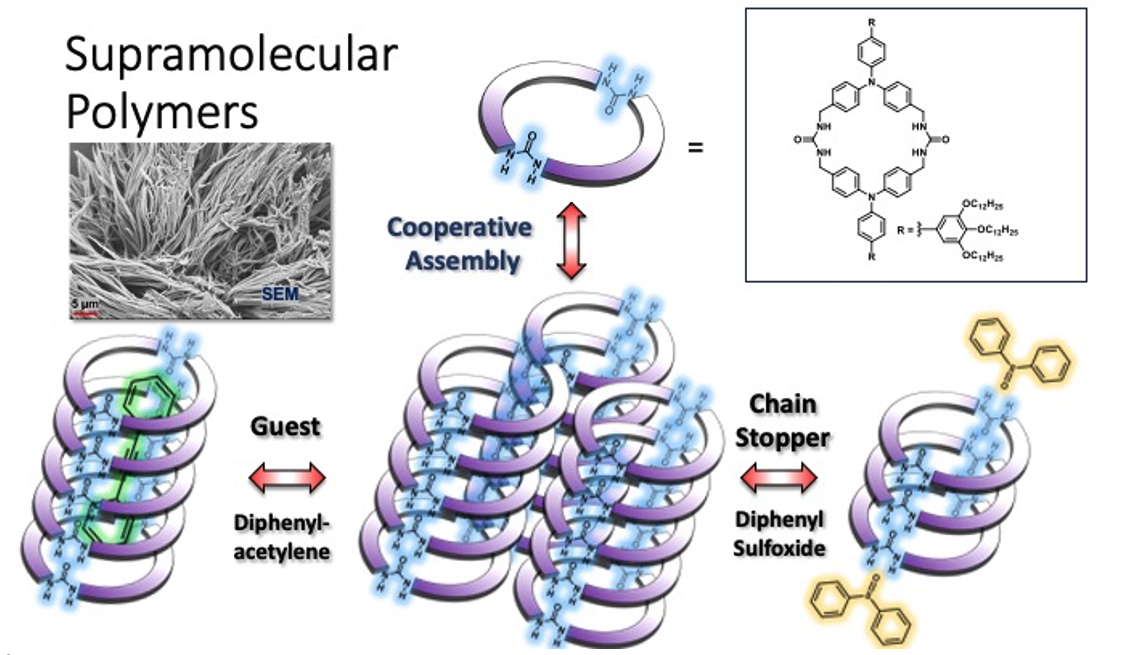
Cooperative Supramolecular Polymerization of Bis-Urea Macrocycles
Bis-urea macrocycles consisting of two urea groups and two rigid C-shaped spacers typically assemble into columnar structures to form porous molecular crystals. These crystals can be used to probe charge-transfer processes with guests or be employed as nanoreactors to facilitate the reactions of encapsulated guests.1,2 In this talk, we examine the assembly of soluble bis-urea macrocycles with exterior tridodecyloxy benzene groups to form supramolecular polymers3 and gels4 and compare them to their crystalline derivatives. Comprised of two methylene urea-bridged triphenylamines, macrocycle 1 exhibits concentration-dependent aggregate formation in THF and THF/H2O mixtures as characterized by 1H NMR and DOSY experiments. Its assembly and disassembly processes were further probed by temperature-dependent UV/vis and fluorescence spectroscopy. Thermodynamic analysis of the emission spectra indicates a cooperative self-assembly pathway with distinct nucleation and elongation regimes. In addition, the assembly/disassembly process was examined in the presence of guests, known to fit inside the columnar channels of crystalline derivatives that lack the solubilizing groups. For example, diphenylacetylene (DPA) guests and chain stoppers were found to influenced both the elongation temperature and enthalpy for assembly. Strategies for selecting (co) monomers that may modulate the function and size of these supramolecular systems will be discussed.
References
- Islam, M.D.; Sindt, A. J.; Hossain, M.S.; Ayare, P. J.; Smith, M.D.; Vannucci, A.K.; Garashchuk, S.; Shimizu, L. S. Phys. Chem. Chem. Phys., 2021 23, 23952-23960.
- Islam, M.F.; Adame-Ramirez, E.; Williams, E.; Kittikhunnatham, P., Smith, M. D.; Pellechia, P.J.; Greytak, A. B.; Shimizu, L. S. Macromolecules. 2022, 55, 11013-11022.
- Prakash, R; Islam, M. F., Kothalawala, R. M.; Hossain, M. S.; Smith, M. D.; Shimizu, L.S. Chem.-Eur. J. 2023, 29, e202300698.
- Prakash, R.; Esmaeili, M.; Gbadamosi, F.; Pellechia, P. J.; Sadati, S.; Shimizu, L. S. Macromolecules 2024, 57, 1312-1318.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















