
 19/06/2024
19/06/2024
 12:00 h
12:00 h
- Lecturer: Prof. Ken D. Shimizu
- University: University of South Carolina (USA)
- Sponsored by:

Molecular Balances and Rotors: Molecule-Sized Scientific Instruments
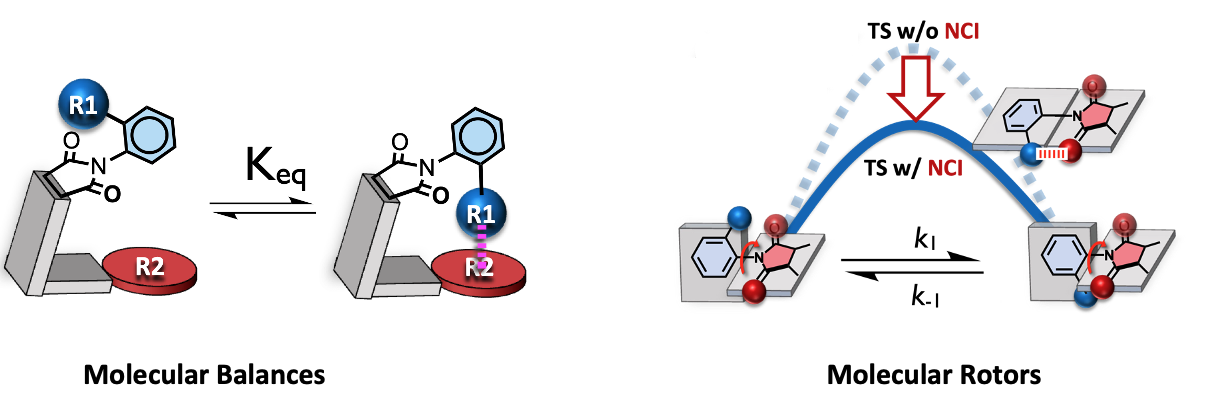
The attractive and repulsive interactions between molecules often define their properties and applications.1 For example, the ability of a drug to find its target or the strength of a Kevlar bullet-proof vest are largely governed by intermolecular interactions. Despite their importance, these interactions are difficult to measure and accurately predict. One reason is that they are very weak: typically, 1/20 to 1/100 the strength of a covalent bond. To address this problem, we developed molecule-sized molecular devices to measure and study the interactions. Our N-phenylimide molecular balances adopt distinct folded and unfolded conformations.[1] Thus, measurement of the folded/unfolded ratio by 1H NMR integration provides an accurate measure of weak intermolecular interactions in the folded conformer.[2] Due to the synthetic versatility and ease of preparation, we have applied this model system to study a range of noncovalent interactions of aromatic surfaces including face-to-face π-stacking, dispersion, OH-π, fluorine-π, heterocyclic π-stacking, and Ag-π. The same N-phenylimide framework can also be used as a molecular rotor to study the kinetic effects of non-covalent interactions. The rate of rotation provides a measure of the ability of the non-covalent interactions to stabilize the bond rotation transition state, providing a model for their influence in enzyme active sites and organocatalyts. We have studied the kinetic effects of hydrogen bonds,[3] pnictogen bonds,[4] and chalcogen bonds and their implications on reactivity.
References:
[1] I. Aprahamian, ACS Cent. Sci. 2020, 6, 347–358.
[2] P. Li, E. C. Vik, K. D. Shimizu, Acc. Chem. Res. 2020, 53, 2705–2714.
[3] E. C. Vik, P. Li, J. M. Maier, D. O. Madukwe, V. A. Rassolov, P. J. Pellechia, E. Masson, K. D. Shimizu, Chem. Sci. 2020, 11, 7487–7494.
[4] B. Lin, H. Liu, I. Karki, E. C. Vik, M. D. Smith, P. J. Pellechia, K. D. Shimizu, Angew. Chem. Int. Ed. 2023, e202304960.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















