06/02/2015
06/02/2015
 12:00 h
12:00 h
 ICIQ Auditorium
ICIQ Auditorium
- Lecturer: Prof. Dr. Marc Koper
- University: Universiteit Leiden (The Netherlands)
- Sponsored by:

Multiple Proton-Coupled Electron Transfer Reactions in Electrocatalysis: Theory vs Experiment
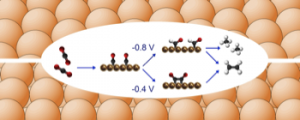
This talk will outline a simple but general theoretical analysis for multiple proton-electron transfer reactions, based on the microscopic theory of proton-coupled electron transfer reactions, recent developments in the thermodynamic theory of multi-step electron transfer reactions, and the experimental realization that many multiple proton-coupled electron transfer reactions feature decoupled proton-electron steps in their mechanism. The theory will be discussed in relation to the experimental results for a number of redox reactions that are of importance for sustainable energy conversion, including oxygen reduction and evolution, and the electrocatalytic reduction of CO2, focusing on their pH dependence and structure sensitivity.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements