 14/03/2018
14/03/2018
 12:00
12:00
- Lecturer: Prof. Andrew D. Smith
- University: EaStCHEM, School of Chemistry, University of St Andrews (UK)
Lewis Base Catalysis: Reaction Development and Heterocycle Synthesis
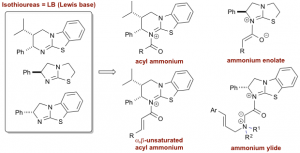
Lewis base-mediated reactions encompass a plethora of molecular transformations. Within this area, we have developed a range of catalytic (asymmetric) processes mediated by a range of Lewis bases.1 This presentation will describe recent developments in the use of isothioureas to promote a number of selective transformations that involve the generation of either an acyl ammonium,2 ammonium enolate,3 a,b-unsaturated acyl ammonium4 or ylide intermediate5 (Figure 1). The application of these methodologies to the (enantioselective) synthesis of a variety of heterocyclic products, as well as mechanistic insights into these processes will be discussed.
Figure 1: Isothioureas and intermediates in enantioselective Lewis base catalysis
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements