Invited Seminar (Group Prof. Arjan W. Kleij) – Prof. Dr. Ivana Fleischer, University of Tübingen, Germany
Workshop 26/04/2024 at 10:30h | ICIQ Library
📍 ICIQ Auditorium
⏰ 12:00 h
There is Something About Chalcogens
Our research interests focus on development of new methods for the synthesis and use of sulfur-containing compounds, such as thioesters and thioethers. They constitute valuable synthetic intermediates and target compounds for material chemistry and pharmaceutical applications.[1] Our aim is to develop efficient transformations employing non-precious metals as homogeneous catalysts.
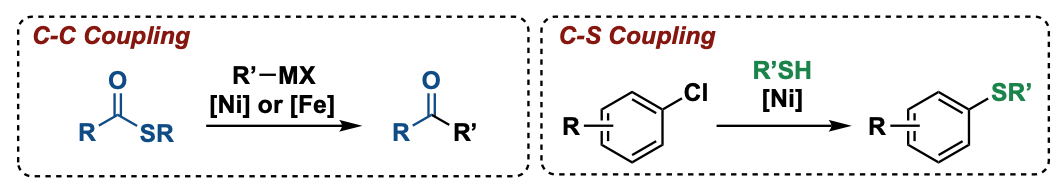
We have demonstrated the usefulness of thioesters in cross coupling reactions with arylzinc reagents to generate ketones.[2] A defined nickel complex was employed as catalyst and a series of functionalized ketones was successfully obtained. The scope was later expanded to the coupling of thioesters with more reactive organomanganese reagents upon iron catalysis.[3]
Furthermore, we developed nickel-catalyzed coupling reactions of challenging aryl chlorides with thiols, whereby max. TOF of 800 h-1 was achieved.[4] A broad scope of substrates containing various functional groups and heterocyclic motifs was successfully converted. A systematic study of couplings of sterically hindered aliphatic thiols was conducted.
Besides, we are interested in the use of alcohols as substrates in transition metal catalyzed reactions, due to their availability and the advantageous atom economy if no preactivation is needed. So far, our research focused on deoxygenation strategies, for example employing a titanium(IV) catalyst.[5]
References
[1] a) K. A. Scott, J. T. Njardarson, Top. Curr. Chem. 2018, 376, 5; b) V. Hirschbeck, P. H. Gehrtz, I. Fleischer, Chem. Eur. J. 2018, 24, 7092.
[2] P. H. Gehrtz, P. Kathe, I. Fleischer, Chem. Eur. J. 2018, 24, 8774.
[3] V. J. Geiger, L. Lefèvre, I. Fleischer, Chem. Eur. J. 2022, 28, e202202212.
[4] a) P. H. Gehrtz, V. Geiger, T. Schmidt, L. Sršan, I. Fleischer, Org. Lett. 2019, 21, 50; b) R. M. Oechsner, J. P. Wagner, I. Fleischer, ACS Catal. 2022, 12, 2233; c) R. M. Oechsner, I. H. Lindenmaier, I. Fleischer, Org. Lett. 2023, 25, 1655; d) I. H. Lindenmaier, R. C. Richter, I. Fleischer, Org. Chem. Front. 2024, DOI: 10.1039/d4qo00260a.
[5] A. Căciuleanu, F. Vöhringer, I. Fleischer, Org. Chem. Front. 2023, 10, 2927.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















