10/05/2019
10/05/2019
 12:00 h
12:00 h
- Lecturer: Prof. Dr. Wolfgang Lubitz
- University: Max-Planck-Institut für Chemische EnergieKonversion (Germany)
- Sponsored by:

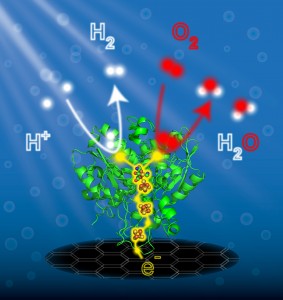
Insight into the Catalytic Cycle of Hydrogenases: Inspiration for the Design of Molecular Catalysts
Hydrogenases catalyze the reversible conversion of hydrogen into protons and electrons at binuclear (NiFe or FeFe) metal centers with very high activity and efficiency. The [NiFe] hydrogenases are quite well understood; important results are briefly reviewed. The active site of [FeFe] hydrogenases is more complex ; it is composed of a classical [4Fe-4S] cluster linked via a cysteine to a [2Fe] center that carries CO and CN– ligands, and a bridging aza-propene dithiolate ligand1. Semisynthetic [FeFe] hydrogenases can be created simply by mixing recombinantly produced enzyme, carrying only the [4Fe-4S] cluster, with chemically synthesized di-iron precursor complexes. By using a variety of chemically distinct or isotopically labeled di-metal complexes, in combination with spectroscopic and electrochemical techniques, crucial insight has been gained into the mechanistic understanding of the catalytic cycle of [FeFe] hydrogenases. It is further demonstrated that the great oxygen sensitivity of these enzymes can significantly be improved by embedding the hydrogenase in a tailor-made polymer matrix. Hydrogenases and related model systems can successfully be attached to electrodes and used in devices.4 All these results are of crucial importance for the future development of catalytic systems for H2 conversion and production.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements