
Modelling the Chemistry of Enzymes: Synthesis and Reactivity of High-Valent Oxoiron Species
The development of high-valent iron-oxo synthetic models that are able to mimic the reactivity of those found in natural iron-oxygenases is an important challenge, as it would enable both a deeper understanding of biological systems and an improvement of artificial oxidation catalysis. While the preparation of iron(IV)-oxo species has significantly evolved over the last two decades, entrapment of iron(V)-oxo compounds has proven much more challenging. In our group, we have been able to trap and spectroscopically characterize iron(V)-oxo-carboxylato species bearing a particular N4 macrocyclic ligand (PyNMe3, Figure 1). This compound is prepared by reaction of the iron(II) precursor with a peracid at very low temperatures. Reactivity studies demonstrate not only that this species is a potent oxidant, but also that it is catalytically relevant as it exhibits selectivity patterns fully congruent with those observed in selective C–H hydroxylation iron catalysis.
Upon changing the nature of the carboxylate ligand in the iron(V)-oxo-carboxylato species, very interesting chemistry is uncovered, which is strongly related to that observed in carboxylic acid directed C-H hydroxylation catalyzed by iron and manganese complexes. Thus, regioselective oxidation of C-H bonds in the g position with respect to the carboxylate moiety is observed, which results in the formation of the corresponding g-lactones. The degree of substitution of the g-carbon atom has also a significant impact on the reaction outcome.
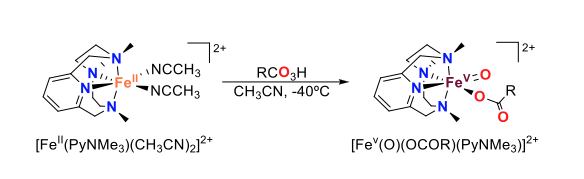
Figure 1. Generation of iron(V)-oxo-carboxylato species by reaction of the iron(II) precursor with a peracid
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements
 01/12/2023
01/12/2023
 12:00
12:00



















