26/11/2018
26/11/2018
 12:00
12:00
- Lecturer: Prof. Javier Garcia Martinez
- University: Universidad de Alicante (Spain)
- Sponsored by:

Discovery and Commercialization of New Family of Catalysts
The development of intracrystalline mesoporosity within zeolites has been a long-standing goal in catalysis as it greatly contributes to alleviate the diffusion limitations of these widely used microporous materials.1 In this contribution, I will present unprecedented insights on the formation of intracrystalline mesoporosity in zeolites by surfactant-templating recently obtained by in situ synchrotron X-ray diffraction and Liquid Cell Transmission Electron Microscopy (Liq-TEM). By combining experimental results and theoretical calculations, the presence of intracrystalline mesoporosity showing local hexagonal order was unambiguously confirmed. Moreover, through the observation of individual zeolite crystals by Liq-TEM, we are able to provide the first time resolved visualization of the formation of mesoporosity in zeolites.2 The presence of this mesoporosity was further evidenced through ex situ gas adsorption, which also confirmed the preservation of most of the microporosity of the zeolites. All these new insights, obtained by combining a number of time-resolved techniques, are an example of the enormous potential of current in situ characterization methods for the rational design of hierarchical zeolites with superior properties and optimal catalytic performance as it has been proved at lab, pilot plant, and industrial scale.
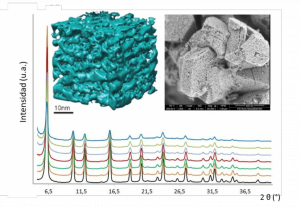
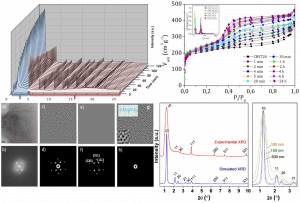 Figure 1. Structural characterization of nanoengineered zeolites showing both crystallinity and mesoporosity as evidenced by X-ray diffraction, transmission electron microscopy and simulation.
Figure 1. Structural characterization of nanoengineered zeolites showing both crystallinity and mesoporosity as evidenced by X-ray diffraction, transmission electron microscopy and simulation.
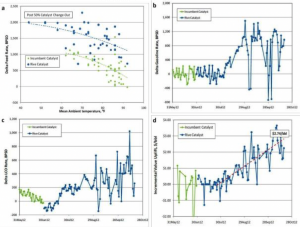
Mesostructured zeolites show of the properties of conventional zeolites including crystallinity, microporosity, strong acidity and hydrothermal stability. The changes in the micropore volume of the mesostructured zeolite Y during the severe hydrothermal treatment steps described herein were similar in trend as those for conventional zeolite Y, but the mesoporosity is preserved even after deactivation. Mesostructured zeolites were also formulated with various matrix compositions into FCC microspheres (of ~ 70 micron in size) by spray drying. The catalysts, after being properly deactivated (e.g. at 788°C under 100% steam for 4 h), were tested on a fixed fluidized bed ACE testing unit with different feedstocks. The catalysts made from mesostructured zeolites produced significantly more gasoline and light cycle oil (transportation fuels), and less bottoms and coke. The improved product selectivity could be attributed to the mesostructure introduced into the zeolites that eased the diffusion limitation in the conventional zeolites.
Figure 2. Microstructure of mesoporous USY zeolite and some refinery data showing the superior catalytic performance of our catalysts in commercial application.
In commercial operation, we observe a combination of improved coke selectivity while increasing feed rate allowed to achieve a significant increase in both LCO and gasoline production. The observed selectivity toward intermediates confirms that the introduction of intracrystalline mesoporosity shortens the diffusion path length and hinders the occurrence of secondary reactions. At the end of a particular commercial trial, the additional value that was delivered to the refinery by replacing the incumbent catalyst with the mesostructured USY containing FCC catalyst was estimated to be over US$2.50/bbl of the FCC feed.3
The commercialization of surfactant-templated zeolites in FCC is an extraordinary example of academic entrepreneurship, which is expected to foster the development of new hierarchical zeolites and their use both in existing processes and new and exciting opportunities.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements