18/02/2015
18/02/2015
 12:00 h
12:00 h
 ICIQ Auditorium
ICIQ Auditorium
- Lecturer: Prof. Dr. Albrecht Berkessel
- University: University of Cologne (Germany)
- Sponsored by:

Carbene Catalysis and the Breslow Intermediate
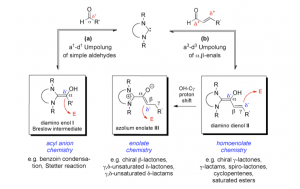
Both in enzymes and in organocatalytic Umpolung, catalysis by N-heterocyclic carbenes hinges on the reversible formation of the so-called Breslow-intermediates [chemically: (di)amino enols] I (Scheme, reaction a), in which the innate polarity of e.g. an aldehyde substrate is inverted from electrophilic to nucleophilic. Postulated by Ronald Breslow in 1958, the first successful generation of diamino enols from aldehydes and carbenes, and their characterization by in situ NMR was reported by us in 2012. In the related a3-d3 Umpolung (“conjugate Umpolung”; Scheme, reaction b), the reaction sequences originate from the interaction of an α,β-unsaturated aldehyde with a carbene. Again, a Breslow-type intermediate is assumed to be pivotal, namely the diamino dienol II. In the latter, the γ-position carries a partial negative charge, and the diamino dienols II consequently react as homoenolate equivalents. A subsequent OH-Cγ proton shift in the diamino dienol II leads to the azolium enolate III. In the latter, the β-position carries a partial negative charge, and the azolium enolates III are therefore considered as enolate equivalents. In sharp contrast to their pivotal character in conjugate Umpolung, no homoenolate II or enolate III appears to have been character- ized until recently.
The lecture will report the generation and characterization, by NMR and X-ray crystal structures, of a number of diamino enols I, of two diamino dienols II and two azolium enolates III, together with NMR monitoring of a diamino dienol (II) to azolium enolate (III) tautomerization. Transformations of the intermediates I–III will be discussed which support the reactivity patterns shown in the Scheme.
References: 1. A. Berkessel, S. Elfert, K. Etzenbach-Effers, J. H. Teles, Angew. Chem. Int. Ed. 2010, 49, 7120-7124. 2. A. Berkessel, S. Elfert, V. R. Yatham, J.-M. Neudörfl, N. Schlörer, J. H. Teles, Angew. Chem. Int. Ed.
2012, 51, 12370-12374. 3. A. Berkessel, V. R. Yatham, S. Elfert, J.-M. Neudörfl, Angew. Chem. Int. Ed. 2013, 52, 11158-11162. 4. A. Berkessel, S. Elfert, Adv. Synth. Catal. 2014, 356, 571–578.
Keywords: reaction mechanism, N-heterocyclic carbenes, Umpolung, Breslow intermediate, NMR, X-ray diffraction.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements