31/05/2017
31/05/2017
 12:00
12:00
 ICIQ Auditorium
ICIQ Auditorium
- Lecturer: Prof. Dr. Robert Wolf
- University: Universität Regensburg (Germany)
Benzylic C−H Bond Oxygenation and Oxidative Chlorination by Flavin Photocatalysis
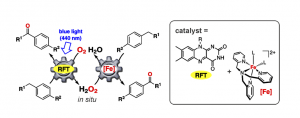
The significance of flavins as photoreceptors and redox cofactors has inspired the use of synthetic flavin analogues as photocatalysts. This talk will describe recent progress in the utilization of the vitamin B2 derivative riboflavin tetraacetate (RFT) for visible-light-driven oxidations. The substrate scope of RFT can be significantly enhanced by metal ion coordination, thereby modifying its reduction potential, as well as by adding catalytically active metal complexes and organic mediators.
Coordination of RFT to scandium(III) ions enables photocatalytic oxygenations of alkylbenzenes with electron-withdrawing substituents, which are hardly feasible using RFT alone. A further improvement of catalyst performance was achieved by combining RFT with a biomimetic iron catalyst. In this system, iron-catalyzed H2O2 disproportionation and alkylbenzene oxygenation crucially contribute to the good overall efficiency.
Finally, a recent extension of RFT photocatalysis to the oxidative chlorination of arenes will be presented. Here, hypochloric acid is produced in-situ by reaction of photocatalytically generated peracetic acid with chloride anions. This allows the chlorination of electron-rich arenes, e.g. anisole, methylanilines, diphenyl ether, and amides, and the α-chlorination of acetophenones.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements