19/05/2017
19/05/2017
 12:00 h
12:00 h
 ICIQ Auditorium
ICIQ Auditorium
- Lecturer: Prof. Declan G. Gilheany
- University: University College Dublin (Ireland)
- Sponsored by:

Asymmetric Grignard Synthesis of Tertiary Alcohols
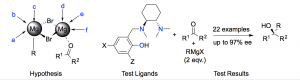
Chiral tertiary alcohols constitute an important class of biologically active molecules.[1] However their asymmetric synthesis remains problematic. An efficient and general enantioselective direct 1,2-addition of organomagnesium reagents to ketones would be extremely desirable but is very challenging. To the best of our knowledge, only two cases have been reported to date where high enantioselectivity was obtained in the absence of metals other than magnesium.[2] The challenges lie in: low enantioface discrimination between the prochiral sides of a ketone, competitive non-stereoselective reaction, enolization/reduction side reactions, competitive radical processes and, most significantly, dynamic processes originating from Schlenk and aggregation equilibria.[3]
We recently used a rational design process based on a mechanistic hypothesis to propose that tridentate ligands should lead to better stereoselection. This was tested with the salan ligands shown. Their use in the asymmetric 1,2-addition of Grignard reagents to ketones allowed generation of chiral tertiary alcohols in high yields and enantioselectivites, with full recovery of the ligand in the work-up.[4] These results will be presented, as will further research with other tridentate ligand types, mechanistic investigations, catalysis studies and expansion of the reaction scope via a design of experiments (DoE) style analysis.[5]
Using this new asymmetric methodology, we implemented a practical solution to the challenging and long-standing problem of enantioselective construction of the C2 stereocentre of the vitamin E suite.[6]
Acknowledgements
This work was supported by the Irish Research Council (GOIP 2011), Enterprise Ireland (CF/2013/3321) and Science Foundation Ireland (12/RC/2275).
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements