28/02/2020
28/02/2020
 12:00
12:00
- Lecturer: Prof. Thomas R. Ward
- University: Universität Basel (Switzerland)
- Sponsored by:

Artificial Metalloenzymes for in vivo Catalysis: Challenges and Opportunities
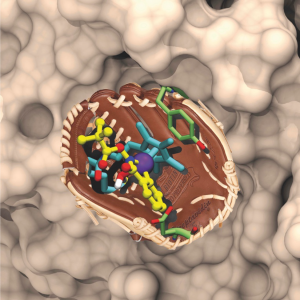
Artificial metalloenzymes result from the incorporation of a abiotic cofactor within a host protein. We and others have been exploiting the potential of the biotin-(strept)avidin technology for the creation of artificial metalloenzymes, Figure. Thanks to the remarkable supramolecular affinity of biotin for either avidin or streptavidin (KD > 10-13 M), linking of a biotin anchor to a catalyst precursor ensures that, upon stoichiometric addition of (strept)avidin, the metal moiety is quantitatively incorporated within the host
protein. Alternatively, human carbonic anhydrase has proven equally versatile for the development of artificial metalloenzymes relying on aryl-sulfonamide anchors to ensure the localization of the abiotic metallocofactors within the host protein.
The resulting artificial metalloenzymes can be optimized either by chemical (variation of the anchorspacer-ligand moiety) or genetic- (mutation of the host protein) means. These chemo-genetic schemes were applied to optimize the performance for twelve different catalyzed transformations as well multiple reaction cascades in the presence of natural enzymes.
More recently, we have been investigating the potential of artificial metalloenzymes for in vivo catalysis to complement metabolic pathways. In this context, E coli’s periplasm as well as surface display have proven particularly versatile to compartmentalize and evolve artificial metalloenzymes while maintaining the critical phenotype-genotype linkage.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements