
 21/02/2025
21/02/2025
 09:15 h
09:15 h
 ICIQ Auditorium Prof. Dr. Kilian Muñiz
ICIQ Auditorium Prof. Dr. Kilian Muñiz
- Lecturer: Prof. Jérôme Waser
- University: EPFL, Switzerland
- Invited by: Prof. Marcos García Suero
Reactive Intermediates from and towards Cyclopropenes
Abstract
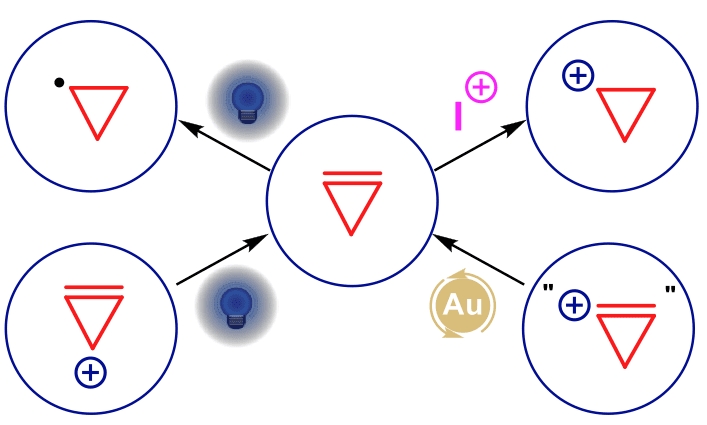
The exploitation of weak bonds and strained rings stands at the center of our research program.1 Combining a ring strain of 55 kcal/mol with a very reactive double bond, cyclopropenes are unique building blocks in synthetic chemistry, giving access to polyfunctionalized cyclopropanes or highly-substituted acyclic systems. The photo-mediated generation of cyclopropyl radicals and their subsequent transformations enables the synthesis of bicyclo[3.1.0]hexanes, polyaromatic compounds and quinolines.2 The electrophilic activation of cyclopropenes leads to highly reactive cyclopropyl cations, initiating rearrangement processes to generate spirocyclic compounds.3 Finally, π-cyclopropenium4 and hypervalent iodine reagents as synthetic equivalents of π-cyclopropeniums,5 using photoredox and gold catalysis, respectively, give access to cyclopropenes with novel substitution patterns.
References
(1) https://www.epfl.ch/labs/lcso/research/
(2) (a) Muriel, B.; Gagnebin, A.; Waser, J. Chem. Sci. 2019, 10, 10716-10722. (b) Muriel, B.; Waser, J. Angew. Chem., Int. Ed. 2021, 60, 4075-4079. (c) Smyrnov, V.; Muriel, B.; Waser, J. Org. Lett. 2021, 23, 5435-5439.
(3) Smyrnov, V.; Waser, J. Org. Lett. 2023, 25, 6999-7003.
(4) Smyrnov, V.; Waser, J. Angew. Chem., Int. Ed. 2024, 63, e202404265.
(5) (a) Li, X.; Wodrich, M. D.; Waser, J. Nat. Chem. 2024, 16, 901-912. (b) Li, X.; Waser, J. J. Am. Chem. Soc. 2024, 146, 29712-29719.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















