Acceleration and Selectivity of 1,3-Dipolar Cycloaddition Reactions Included in a Polar [4 + 2] Octa-imine Bis-calix[4]pyrrole Cage
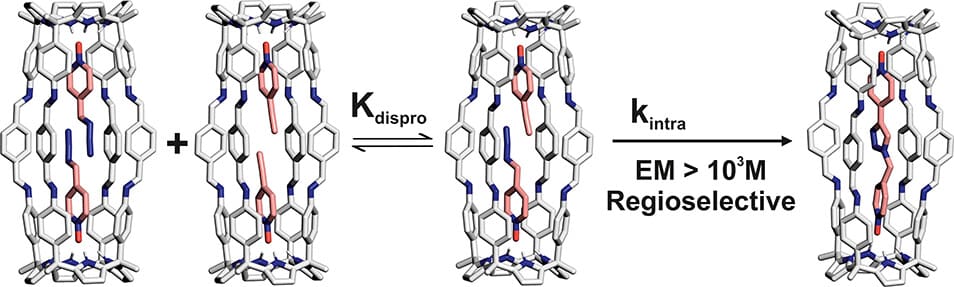
We describe the quantitative self-assembly (>90%) of a [4 + 2] octa-imine cage (1) in a CDCl3:CD3CN 9:1 solvent mixture containing 0.5% of acetic acid. Cage 1 is based on two identical aryl-extended calix[4]pyrrole units linked through eight dynamically reversible imine bonds. Cage 1 forms thermodynamically and kinetically highly stable inclusion complexes featuring 1:1 and 2:1 stoichiometry with suitable para-substituted pyridine-N-oxides. The ability of 1 for the pairwise inclusion of two different pyridine-N-oxides led us to investigate its properties as a reactor vessel. The coinclusion of 4-azido pyridine-N-oxide and 4-ethynyl pyridine-N-oxide did not produce a detectable acceleration of their 1,3-dipolar cycloaddition reaction. Conversely, the coinclusion in cage 1 of the same alkyne dipolarophile with 4-azido(alkyl) pyridine-N-oxides (alkyl= methyl, ethyl) produced significant reaction acceleration. We quantified the reactions’ acceleration with an effective molarity (EM) of ∼103 M, corresponding to the more prominent reported value of a bimolecular 1,3-dipolar cycloaddition reaction in a molecular vessel by directly detecting the ternary Michaelis complex. The included reactions are quantitative and regioselective, yielding exclusively the 1,4-disubstituted triazole isomers. We propose that the selectivity of 1 in accelerating the included 1,3-dipolar cycloadditions is related to (a) the entropy gain provoked by the reaction’s inclusion, (b) the rigidity of the container, and (c) the spatial fixation of the polar knobs (pyridine-N-oxide) carrying the reacting groups in its two functionalized hemispheres. The two latter characteristics render the distance between the reacting groups (azido and ethynyl) almost fixed by design, thus allowing or not achieving the transition state’s geometry. We support our hypothesis with the help of DFT calculations of the inclusion complexes’ structures.
Li, Y.; Mirabella, C. F. M.; Aragay, G.; Ballester, P.
JACS Au 2025
DOI:
10.1021/jacsau.4c01118
Associated projects:
-
MOCACUBE
This project aims to synthesizing unprecedented covalent molecular clefts and cavitands, as well as self-assembling large molecular cages based on dynamically covalent imine bonds. The transformation of post-synthesized imine cages into covalent derivatives will also be pursued. We will prepare different versions of the container compounds to be soluble in organic solvents and in water. All compounds share in common having large aromatic cavities equipped with convergent functional groups deriving from aryl-extended calix[4]pyrrole units. In this way, they act as molecular containers for polar substrates. The cavities volumes are adequate for the inclusion of one or two sizable molecules or ions. On the one hand, cavitands possess cavities open only at one end that define their upper rims.
See more -
Química Supramolecular Interdisciplinar
The scientist in charge of the group, Prof. Pau Ballester, is an ICREA professor with over 30 years of experience in the field of supramolecular chemistry. The group is an international reference in the study of supramolecular assemblies, molecular recognition, and supramolecular catalysis. The group's results have led to more than 270 scientific articles, 9 book chapters, and three patents.
See more

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements






















