Nickel Dynamics Switches the Selectivity of CO2 Hydrogenation
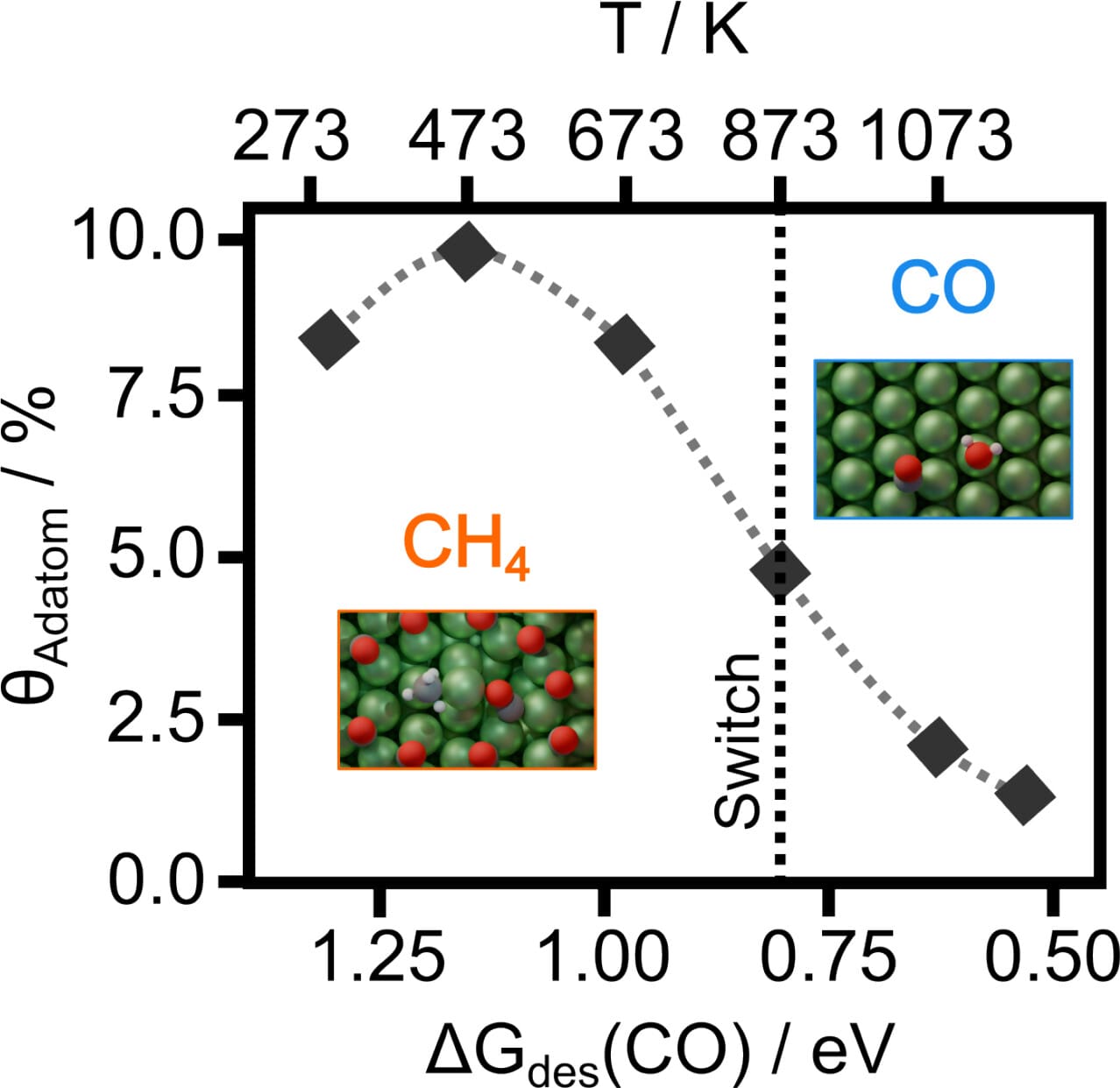
The Reverse Water Gas-Shift reaction (CO2+H2 CO+H2O) allows to balance syn-gas under industrial conditions. Nickel has been suggested as a potential catalyst but the temperature required is too high, more than 800 degrees C, limiting practical implementation but when lowering the temperature methanation occurs. Simulations via Density Functional Theory on well-defined surfaces have systematically failed to reproduce these experimental results. But under reaction conditions, Ni surfaces are not static and DFT models coupled to microkinetics show that low temperatures (high CO coverages) drive the generation of Ni adatoms that are the active sites for methanation. At higher temperatures, the adatom population decreases, and the selectivity towards CO increases. Thus the mechanism behind the selectivity switch is driven by the dynamics induced by reaction intermediates. Our work contributes to the inclusion of dynamic aspects of materials under reaction conditions in the understanding of complex catalytic behaviour.
CO+H2O) allows to balance syn-gas under industrial conditions. Nickel has been suggested as a potential catalyst but the temperature required is too high, more than 800 degrees C, limiting practical implementation but when lowering the temperature methanation occurs. Simulations via Density Functional Theory on well-defined surfaces have systematically failed to reproduce these experimental results. But under reaction conditions, Ni surfaces are not static and DFT models coupled to microkinetics show that low temperatures (high CO coverages) drive the generation of Ni adatoms that are the active sites for methanation. At higher temperatures, the adatom population decreases, and the selectivity towards CO increases. Thus the mechanism behind the selectivity switch is driven by the dynamics induced by reaction intermediates. Our work contributes to the inclusion of dynamic aspects of materials under reaction conditions in the understanding of complex catalytic behaviour.
González, J.M.; Sabadell-Rendón, A.; Kazmierczak, K.; Euzenat, F.; Montroussier, N.; Curulla-Ferré, D.; López, N.
Angew. Chem.-Int. Edit. 2024,
DOI:
10.1002/anie.202417392

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















