Nickel/Copper Catalyzed C-C and C-N bond Formation Reactions to Forge sp3 Carbon Linkages
Transition metal-catalyzed cross-coupling reactions have become indispensable tools for forging carbon-carbon and carbon-heteroatom bonds. These approaches have been widely applied to the preparation of biologically-relevant molecules and functional materials in both academic and industrial laboratories. Aimed at improving the applicability and practicality of these venerable reactions even further, chemists have been challenged to come up with new cross-coupling synthons that operate under ambient conditions, thus increasing the flexibility in synthetic design when accessing densely functionalized molecules. This Doctoral Thesis focuses on the development of new techniques to use ketones and unactivated halides in cross-coupling reactions.
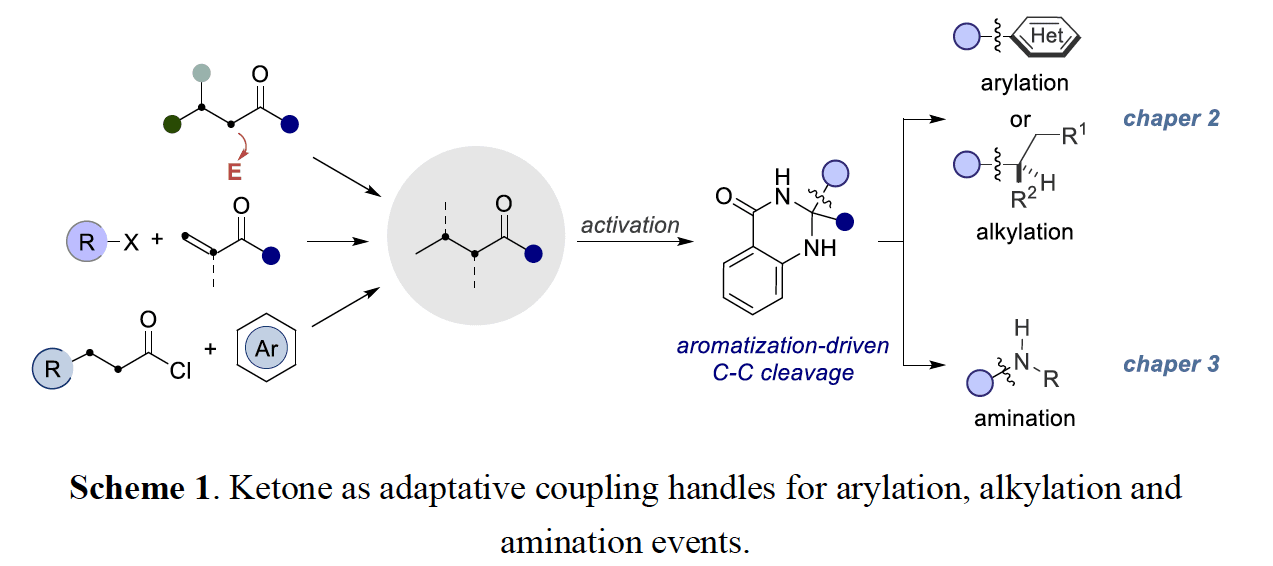
The first section focuses on the development of a photoredox/nickel dual catalytic strategy for forging C(sp2)–C(sp3) and C(sp3)–C(sp3) architectures from dihyquinazolinones. At the outset of this work, transition metal catalyzed ketone α C– C bond activation was particularly limited to strained structures, utilization of directing groups, high-temperatures, or a combination of the preceding. This methodology takes advantage of ketone derived dihyquinazolinones as adaptive one electron handles in combination with the flexibility and modularity of nickel catalysis, allowing for abundant ketones to be formally used as cross-coupling synthons with aryl and alkyl bromide electrophiles. Preliminary mechanistic experiments suggest a reductive quenching photoredox cycle, initiated by single electron oxidation of the dihyquinazolinones by the excited state of the photocatalyst.
The following chapter focuses on the use of dihyquinazolinones in copper catalyzed C(sp3) amination reactions as a de novo strategy for accesing aliphatic amine architectures from simple ketone derivatives. This protocol is distinguished by its mild reaction conditions, wide substrate scope and controllable regioselectivity, thus setting the basis to apply this technique in advanced building blocks. Preliminary mechanistic investigations indicate the presence of radical intermediates and a mode of action by which tert-butoxyl radical is involved in the activation of the dihyquinazolinone.
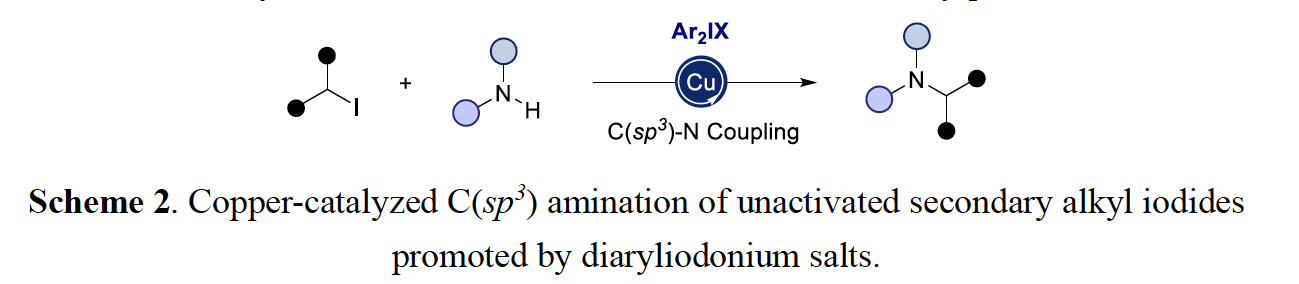
The last research section deals with a copper-catalyzed C(sp3) amination of unactivated secondary alkyl iodides promoted by diaryliodonium salts. This technique takes advantage of diaryliodonium salts to generate aryl radicals in the presence of amido-Cu(I) complexes, resulting in halogen atom abstraction of an alkyl iodide prior to sp3 C–N reductive elimination. This method is characterized by its mild reaction conditions and excellent chemoselectivity while offering a complementary protocol to the preparation of aliphatic amines.

If you would like to follow the ceremony on ZOOM, please, register here.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements
 16/10/2023
16/10/2023
 10:45
10:45
 ICIQ Auditorium
ICIQ Auditorium



















