ICIQ – Crysforma
Pharmaceutical solid state services


Crysforma provides complete scientific support for the discovery, analysis and scale-up of polymorphs, hydrates, amorphous phases, salts and co-crystals of active pharmaceutical ingredients or intermediates.
The main working areas are:
- The modification of the solid state properties of active compounds to obtain the most suitable for the desired application (polymorphism, salts and co-crystal screenings).
- The optimisation of crystallisation processes to improve the development stages and to obtain the solids with the desired properties (purity, particle size, polymorph, morphology,…).
- The characterisation of the solid state of drug substance and drug product (PXRD, SCXRD, microED, DSC, TGA, DVS, PSD, IR, Raman, NMR, synchrotron…).
- Supporting the IP protection of new generated solid forms.
Crysforma has developed its own crystallisation screening methodology based on the combination of several crystallisation techniques and the individual monitoring of the experiments by highly skilled scientists to maximise the information extracted from each crystallisation. Our projects are always carried out in close collaboration with the client, adapted to the customer’s needs and under strict confidentiality conditions.
Expertise
Diastereomeric salts and chiral co-crystals formation
Most drug candidates have chiral centres in their structure. Generally only one of the enatiomers has got the sought-after biological activity, thus a separation of both enantiomers is necessary to have a higher activity in lower doses and to avoid regulatory issues, as the presence of the other enantiomer may sometimes lead to undesired secondary effects.
Crysforma has successfully applied its in-house developed salt screening methodology to the resolution of chiral molecules with ionization sites through diastereomeric salt formation. In a similar way, non-ionisable chiral molecules can also be resolved by formation of co-crystals with chiral coformers.
Type of studies:
- Crystallisation screening to discover the optimal chiral resolution agent and crystallisation solvent.
- Scale-up of the resolution process.
- Development of HPLC analytical methods to determine the enantiomerical excess.
- Determination of absolute configuration by single crystal X-ray diffraction of enantiomerically pure compounds.
Discovery, characterisation and scale-up of pharmaceutical co-crystals
Crysforma offers systematic co-crystal screening studies to discover and select the co-crystal of an API with optimal solid state properties.
In recent years, the development of a pharmaceutical co-crystal has become a novel strategy to improve the solid state properties of an API. Co-crystals are an alternative to salts when these do not have the appropriate solid state properties or cannot be formed due to the absence of ionizable sites in the API.
Crysforma has developed its own co-crystal screening methodology adequate for co-crystal formers with very different solubilities. The methodology combines several crystallisation procedures specifically useful to generate co-crystals, as solid state grinding, solvent evaporation, reaction crystallisation and crystallisation from the melt.
Type of studies:
- Co-crystal screening of pharmaceutical ingredients with selected co-crystal formers
- Characterisation of pharmaceutical co-crystals
- Development of reliable preparation procedures
- Scale-up of the selected co-crystal
Crystallisation of pharmaceutical compounds
Crysforma’s extensive experience in crystallisation of pharmaceutical compounds is used to successfully address complex crystallisation challenges related to crystallisation of poorly crystalline compounds, crystallisation scale-up, or optimisation of crystallisation processes to improve yield, purity or crystallinity and to obtain the desired polymorph controlling the particle size distribution to fulfil the needs of the industrial process.
Moreover, a unique combination of expertise and state-of-the-art instrumentation allows the structure determination of all types of small and medium sized molecules by single crystal X-ray diffraction (SCXRD) using crystals of reduced dimensions.
Type of studies:
- Crystallisation of compounds that are difficult to crystallise or that are formerly only known as amorphous solids
- Development and scale-up of robust crystallisation procedures
- Optimisation of crystallisation processes (yield, desired polymorph, purity, particle size, crystallinity, etc.)
- Crystallisations oriented to the generation of single crystals for SCXRD structure determination
- SCXRD structure determination of pharmaceutical organic molecules and natural products, which includes the absolute configuration determination of enantiomerically pure compounds.
Discovery, characterisation and scale-up of polymorphs
Crysforma offers complete scientific support to discover and characterise the most relevant polymorphs, hydrates and amorphous phases of a drug and to select the candidate with optimal solid state properties.
Polymorphism, the ability of a chemical to crystallise in more than one crystalline phase, is a key issue in the pharmaceutical industry. Different polymorphs can have different physical and chemical properties, and thus can have an influence on the bioavailability of the pharmaceutical compound.
Additionally, polymorphism is key to patent strategy, as different polymorphs can give rise to independent IP claims. Moreover, the relative stabilities of the different polymorphs must be known in order to avoid unwanted polymorph transformations during production of the active pharmaceutical ingredient or in the final pharmaceutical compound.
Crysforma has developed its own crystallisation screening methodology based in the use of several crystallisation procedures, oriented to obtain the thermodynamically stable phase as well as kinetically favoured phases. Moreover, solvent-mediated as well as solvent-free crystallisation procedures are assayed.
Type of studies:
- Initial or fast polymorphism screening for early stages candidates
- Comprehensive polymorphism study of a drug substance
- Determination of the relative stability of different polymorphs
- Scale-up of selected polymorphs
- Development of analytical methods for polymorph quantification
- Fast powder X-ray diffraction analysis characterisation
Powder X-Ray Diffraction Services
Polymorph quantification and detection limit determination
Development of analytical methods for the quantification of a given polymorph in a mixture and the determination of the limit of detection (LOD). Crysforma has developed a measurement procedure to be able to quantify a given polymorph and determine its presence with very low detection limits (typically in the range of 0.3 and 1%, depending on the API). The same procedure can be applied in mixtures of different crystalline products (API 1 + API 2, API + excipient, etc).
Fast analysis service
Crysforma offers a fast and reliable powder X-Ray diffraction service, including several analysis types:
- Crystalline phase identification, comparing against standards defined by the company.
- Measurements according to specific analytical methods developed by Crysforma.
- Component search in specialised databases.
Variable temperature PXRD
On its own or in combination with other thermal analysis techniques, VT-PXRD can provide useful information regarding crystal structure, dehydration / desolvation, phase transitions, melting and decomposition of pharmaceutical compounds.
When more powerful instrumentation is necessary
Sometimes the standard and most available analytical instruments are not enough to get the needed intensity and sensitivity required to solve specific analytical problems and more powerful instrumentation is necessary to obtain the answers.
The radiation based in the synchrotrons is amongst the most powerful in instrumental analysis and is becoming fundamental in many industrial applications, particularly in the characterisation of the solid state of APIs and final drug products. This radiation can be applied in a broad range of X-ray techniques improving the signal intensity, sensitivity and definition.
CRYSFORMA is now offering analysis using the ALBA synchrotron facilities to bring this high technology closer to the industry to solve the problems that cannot be solved in conventional equipment.
The intensity, resolution and signal definition obtained in the analysis performed using the high energy radiation of a synchrotron makes it of special interest in the following issues:
- Differentiation of two very similar polymorphs of an API.
- Lowering the limit of detection of a polymorph of an API or drug product.
- Detection and quantification of determined polymorphs in complex mixtures, for example of a formulated drug product.
- Structure elucidation based on powder X-ray diffraction.
Discovery, characterisation and scale-up of crystalline salts
Crysforma offers systematic salt screening studies to discover and select the salt derivative of an API with optimal solid state properties.
Preparation of a salt is the classical strategy to optimise the solid state properties of an active pharmaceutical ingredient. Salt derivatives can improve crystallinity, solubility and stability of a pharmaceutical compound, and are often chosen instead of the free acid or base.
Crysforma has developed its own salt screening methodology based in the use of selected crystallisation procedures in an optimised group of crystallisation solvents. The methodology allows performing 1000 crystallisation experiments from 10 g of starting API under controlled conditions.
Type of studies:
- Salt screening of pharmaceutical ingredients with selected counterions
- Characterisation of crystalline salts
- Development of reliable preparation procedures
- Scale-up of the selected salt
Solubility screening
Solubility screening studies provide information on the best solvent media for a given crystallisation process. Solubility data is also relevant during pre-formulation evaluations of APIs. Solubility curves and metastable zone width in a given solvent are determined with small amount of API and in a short period of time.
Intrinsic dissolution
An additional characterisation of bulk drug substances and excipients is the measurement of intrinsic dissolution rates. Its determination can be in some cases important since bioavailability of an API is influenced by the dissolution rate. Furthermore, intrinsic dissolution tests are a way to prove chemical purity, batch-to-batch consistency and sameness after changes in production.
Amorphous content
Mechanical stresses during processing may introduce small amounts of amorphous material which can have a relevant effect on the products properties, including solubility and stability. Crysforma applies several available analytical techniques (PXRD, thermal analysis, DVS) to determine small amounts of amorphous phase in a crystalline solid.
Solid form hygroscopicity and stability
Use of dynamic vapour sorption analysis (DVS) and climatic chambers to determine the effects of relative humidity and temperature on the solid form of interest.
Particle size distribution
A very important characteristic of a solid to understand its physical and chemical properties is the particle size. The particle size distribution of an API can be crucial in order to have the required solubility parameters within the specification of the product. Therefore, a standardised analysis is needed in order to determine the PSD of a production batch. Crysforma develops analysis methods and performs the analyses of samples using a laser diffraction methodology (wet dispersion).
Physisorption gas analysis: Surface area and porosity measurement (BET analysis)
The specific surface area and the porosity are important parameters for the characterisation of solids. The physical and chemical behaviour of solids can be studied and directly correlated to the pore size and distribution of the material. BET analysis provides this information by evaluating the nitrogen or helium gas adsorbed at the surface of the solid.
Single crystal structure determination
SCXRD structure determination of pharmaceutical organic molecules and natural products, which includes the absolute configuration determination of enantiomerically pure compounds. A crystallisation screening can also be performed if no single crystals are available.
Tailored training sessions on request
Crysforma offers training services in pharmaceutical solid state development:
Workshops
Tailor-made training courses at the customer’s request (in crystallisation, a given analytical technique etc.)
Equipment
-
Dynamic Vapor Sorption
Determine the effects of relative humidity and temperature on the solid form of interest in combination with a climatic chamber and the powder X-ray diffraction analysis at variable relative humidity.
-
Electron diffractometer

Elucidation of the structure and determination of the absolute configuration with a very small amount of material and “nano” crystals.
-
Particle size distribution
Development and validation of analytical methods to determine the PSD of samples using a laser diffraction methodology (wet dispersion).
-
Powder X-Ray diffractometer
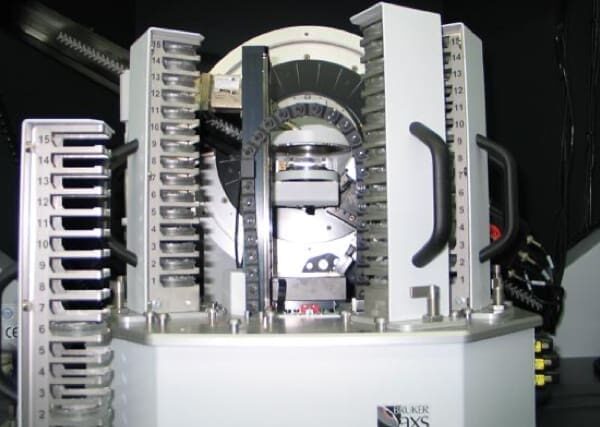
Crystalline form quantification and detection limit determination. Fast analysis service. Variable humidity and temperature analysis. Analysis of drug substance and drug product.
-
Single crystal X-Ray diffractometer
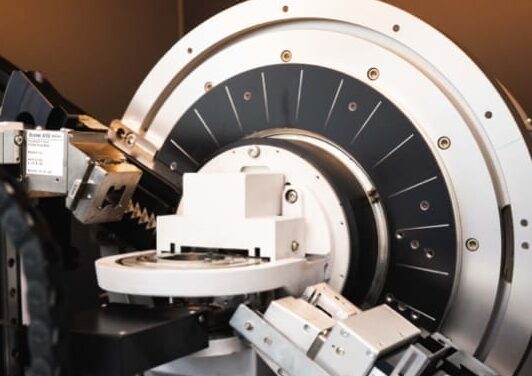
Elucidation of the structure and determination of the absolute configuration. Crystallisation screenings to grow up suitable single crystal for analysis.
-
Thermal analysis

Differential scanning calorimetry, thermogravimetric analysis, hot-stage microscopy to characterise the thermal process of a given sample.

Get started with an expert
Together, let’s create a brighter future providing solutions through a partnership
Connect with us














