The secrets of phenolphthalein
Objective: See how an indicator can have different color changes

-
Laboratory materials
Beakers
Pipettes
Tests tubes
Test tubes rack
-
Reagents
Phenolphthalein (prepared solution: 0.1 g in 60 mL of ethanol and 40 mL of water)
Sodium hydroxide (NaOH)
Chlorhydric acid 2 M (HCl)
Water
-
Safety
Don't forget the gloves, lab coat, and safety goggles!!!
-
Questions
How is the sodium hydroxide solution prepared?
What is observed in the third tube? Why?
Procedure
- Prepare 100 mL of a 0.5 M sodium hydroxide solution.
- Add a few drops of phenolphthalein solution to the sodium hydroxide solution until a vibrant pink color is observed. Then, fill three test tubes halfway with this solution.
- In the first test tube, leave it as a control.
- In the second test tube, add the hydrochloric acid solution drop by drop until the pink color disappears.
- In the third test tube, add 2-3 small pieces of solid sodium hydroxide and shake the solution to dissolve them. Observe what happens.
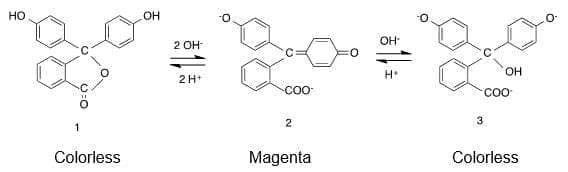
Theoretical explanation
The ‘normal’ behavior of phenolphthalein is to exhibit a pink color in basic solutions and be colorless in acidic solutions. However, phenolphthalein can also become colorless in more concentrated basic solutions.
In acidic solution, phenolphthalein adopts the form 1, which is colorless. With the addition of sodium hydroxide, two protons are lost to produce the red-colored dianion 2, while further basic addition yields form 3, which is also colorless.
















