It does not hurt
Objective: To calculate the concentration of active iodine in an iodine-based disinfectant

-
Laboratory materials
Erlenmeyer
Graduated cylinder
Burette
Beaker
-
Reagents
Betadine®, 10 mL
Sodium thiosulfate pentahydrate 0.1 M (Na2S2O3·5H2O)
Water
-
Safety
Don't forget the gloves, lab coat, and safety goggles!!!
-
Questions:
How is it possible to know when the titration is done? Why there is no need of indicator?
Which reaction takes place?
Calculate the concentration of active iodine present in the Betadine® and compare it with the data provided in the bottle.
Procedure
- Place 10 g of Betadine® in an Erlenmeyer flask and record the exact mass.
- Add 25 mL of water and titrate it with 0.1 M sodium thiosulfate.
- Note the volume of sodium thiosulfate consumed.
Theoretical explanation
Betadine® contains polyvinylpyrrolidone iodine (PVP-I), which is a polymer that releases iodine (I2) upon contact with water.
During the titration, as the iodine from Betadine® comes into contact with sodium thiosulfate, a redox reaction occurs where iodine is reduced to iodide and thiosulfate is oxidized to tetrathionate. The adjusted chemical reaction is as follows:
I2 + 2 S2O32- 2 I– + S4O62-
Given that we know the volume of Betadine® that we have titrated (10 mL), the concentration of thiosulfate (0.1 M), and the volume of thiosulfate used to reduce all the iodine (reading from the burette), the concentration of iodine in Betadine® can be calculated using this formula:
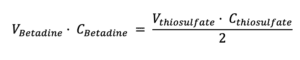
Knowing the concentration in moles per liter (M), the concentration in mass (g/L or mg/mL) can be obtained by considering that the molecular weight of iodine is 254 g/mol.
No indicator is needed because iodine itself serves this function. The solution changes from brown to colorless.















