Citric acid in juices
Objective: To determine the citric acid in freshly prepared orange juice and in commercial juice (it can be also performed with lemon juice)

-
Laboratory materials
Erlenmeyer
Beakers
Burette
Fruit juicer
-
Reagents
Oranges
Lemons
Commercially available orange/lemon juice
Phenolphthalein
Sodium hydroxide 0.5 M (NaOH)
Water
-
Safety
Don't forget the gloves, lab coat, and safety goggles!!!
-
Questions
Which reaction takes place during the titration?
Determine the concentration of citric acid in all juices. Which conclusions can be reached?
Procedure
- Squeeze the orange and the lemon and store the juices in two beakers.
- Add 10 mL of orange juice into an Erlenmeyer flask and then add 25 mL of water and a few drops of phenolphthalein.
- Titrate this solution with 0.5 M sodium hydroxide.
- Repeat the process with 10 mL of freshly prepared lemon juice.
- Finally, repeat the process with the commercial juices and compare the results.
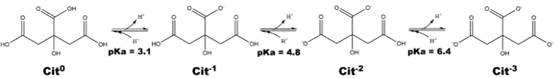
Theoretical explanation
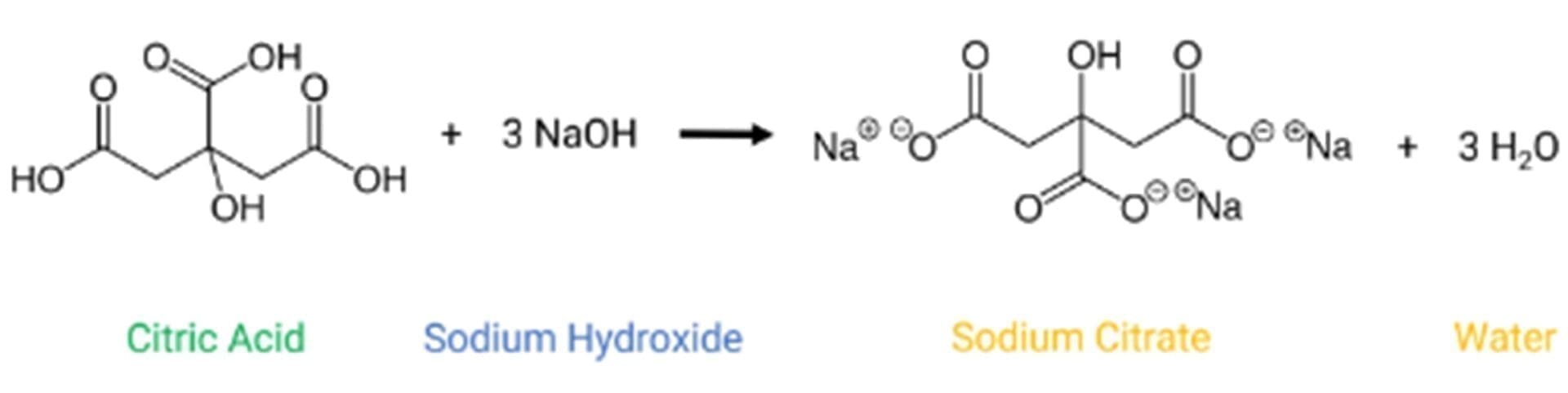
It is well-konwn that orange and lemon juices are acidic. The main chemical compound responsible for this acidity is citric acid, a triacid carboxylic acid that is produced by the fermentation of sugars (>106 tons per year) for use in beverages and food (70%), detergents (20%), and cosmetics, pharmaceuticals, and other applications (10%).
In contact with water, the deprotonation of the three carboxylic acids results in three acid/base equilibria, each with its acidity constant (Ka):
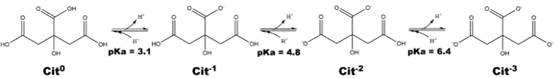
When titrating citric acid with NaOH, the pH of the solution becomes more basic and the acids are deprotonated. Once we have added just enough base to deprotonate all the acids (forming Cit3- in the diagram), the next drop of NaOH causes the pH of the solution being titrated to rise sharply from 7 to over 9. This pH change causes phenolphthalein to turn pink, as its endpoint pH is around 8-8.5. At this point, the titration is considered complete.
Since we know the volume of 0.5 M NaOH we have used, we can calculate the moles of base needed to neutralize the citric acid present in the juice. Knowing that the stoichiometric ratio between the triacid and the base is 1:3, we can calculate the moles of citric acid present in the sample. Finally, we can use the molecular weight of citric acid (192.124 g/mol) to calculate the mass.
The chemical reaction is: