Bouncy ball
Objective: Prepare a bouncy ball

-
Laboratory materials
1 plastic beakers
Glass rod
Graduated cylinders
-
Reagents
Sodium silicate (Na2SiO3)
Ethanol
Water
Food coloring
-
Safety
Don't forget the gloves, lab coat, and safety goggles!!!
-
Questions
Which type reaction is taking place?
Procedure
- Add 20 mL of sodium silicate solution into a plastic cup and add a drop of food coloring.
- Add 5 mL of ethanol and stir with the glass rod, making circular movements until obtaining a soft solid.
- Take the solid with the palm of your hand (wearing gloves) and shape it into a ball, being careful not to let it fall apart. Occasionally, we can moisten the mixture with a little water.
- NOTE: Be careful not to mix the test tubes. One for ethanol and the other for silicate.
Theoretical explanation
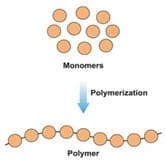
When sodium silicate combines with ethanol, the silicate molecules (monomers) begin to bond together, forming chains that we call polymers. This reaction is called polymerization. The chains formed eventually cross-link, forming three-dimensional networks that cause the material to harden and transition from a liquid to a solid.