An Electrocatalytic Cascade Reaction for the Synthesis of Ketones Using CO2 as a CO Surrogate
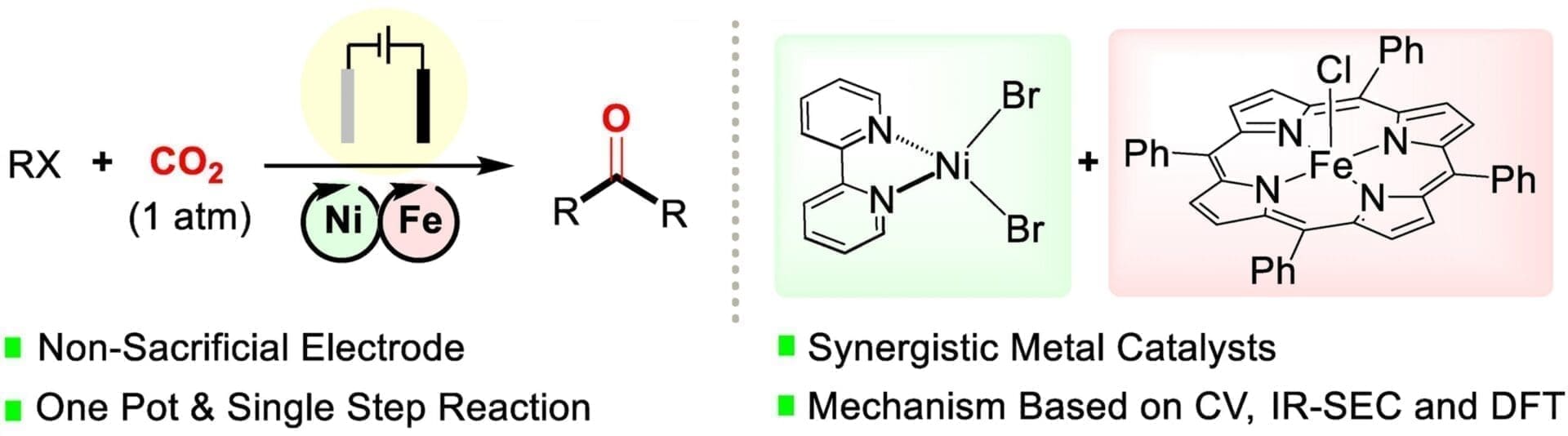
The construction of carbonyl compounds via carbonylation reactions using safe CO sources remains a long-standing challenge to synthetic chemists. Herein, we propose a catalyst cascade Scheme in which CO2 is used as a CO surrogate in the carbonylation of benzyl chlorides. Our approach is based on the cooperation between two coexisting catalytic cycles: the CO2-to-CO electroreduction cycle promoted by [Fe(TPP)Cl] (TPP=meso-tetraphenylporphyrin) and an electrochemical carbonylation cycle catalyzed by [Ni(bpy)Br2] (2,2′-bipyridine). As a proof of concept, this protocol allows for the synthesis of symmetric ketones from good to excellent yields in an undivided cell with non-sacrificial electrodes. The reaction can be directly scaled up to gram-scale and operates effectively at a CO2 concentration of 10 %, demonstrating its robustness. Our mechanistic studies based on cyclic voltammetry, IR spectroelectrochemistry and Density Functional Theory calculations suggest a synergistic effect between the two catalysts. The CO produced from CO2 reduction is key in the formation of the [Ni(bpy)(CO)2], which is proposed as the catalytic intermediate responsible for the C−C bond formation in the carbonylation steps.
Sheta, A. M.; Fernandez, S.; Liu, C.; Dubed-Bandomo, G. C.; Lloret-Fillol, J.
Angew. Chem.Int. Ed. 2024, e202403674
DOI:
10.1002/anie.202403674

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















