Influence of the solvent in the self-assembly and binding properties of [1 + 1] tetra-imine bis-calix[4]pyrrole cages,
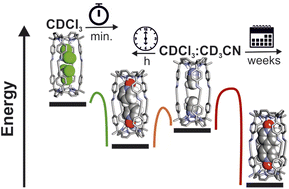
We report the self-assembly of shape-persistent [1 + 1] tetra-imine cages 1 based on two different tetra-α aryl-extended calix[4]pyrrole scaffolds in chlorinated solvents and in a 9 : 1 CDCl3 : CD3CN solvent mixture. We show that the use of a bis-N-oxide 4 (4,4′-dipyridyl-N,N′-dioxide) as template is not mandatory to induce the emergence of the cages but has a positive effect on the reaction yield. We use 1H NMR spectroscopy to investigate and characterize the binding properties (kinetic and thermodynamic) of the self-assembled tetra-imine cages 1 with pyridine N-oxide derivatives. The cages form kinetically and thermodynamically stable inclusion complexes with the N-oxides. For the bis-N-oxide 4, we observe the exclusive formation of 1 : 1 complexes independently of the solvent used. In contrast, the pyridine-N-oxide 5 (mono-topic guest) produces inclusion complexes displaying solvent dependent stoichiometry. The bis-N-oxide 4 is too short to bridge the gap between the two endohedral polar binding sites of 1 by establishing eight ideal hydrogen bonding interactions. Nevertheless, the bimolecular 4⊂1 complex results as energetically favored compared to the 52⊂1 ternary counterpart. The inclusion of the N-oxides, 4 and 5, in the tetra-imine cages 1 is significantly faster in chlorinated solvents (minutes) than in the 9 : 1 CDCl3 : CD3CN solvent mixture (hours). We provide an explanation for the similar energy barriers calculated for the formation of the 4⊂1 complex using the two different ternary counterparts 52⊂1 and (CD3CN)2⊂1 as precursors. We propose a mechanism for the in–out guest exchange processes experienced by the tetra-imine cages 1.
Mirabella, C. F. M.; Aragay, G.; Ballester, P.
Chem. Sci. 2022, (14), 186-195
DOI:
10.1039/D2SC05311J

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















