Click azide-alkyne cycloaddition for the synthesis of D-(–)-1,4-disubstituted triazolo-carbanucleosides
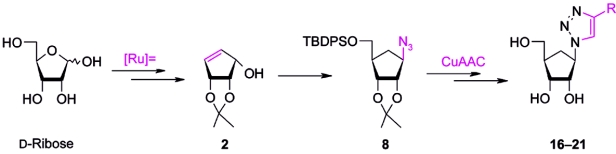
A revisited and improved synthesis of an optically active azido-carbanucleoside is reported. This azido precursor is used in the successful and versatile synthesis of enantiomerically pure D-(-)-1,4-disubstituted 1,2,3-triazolo-carbanucleosides via copper(I)-catalyzed and microwave-assisted Huisgen 1,3-dipolar cycloaddition.
J. Broggi, H. Kumamoto, S. Berteina-Raboin, S. P. Nolan, L. A. Agrofoglio
Eur. J. Org. Chem. 2009, 1880-1888

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements


















