Active and Selective Ensembles in Oxide-Derived Copper Catalysts for CO2 Reduction
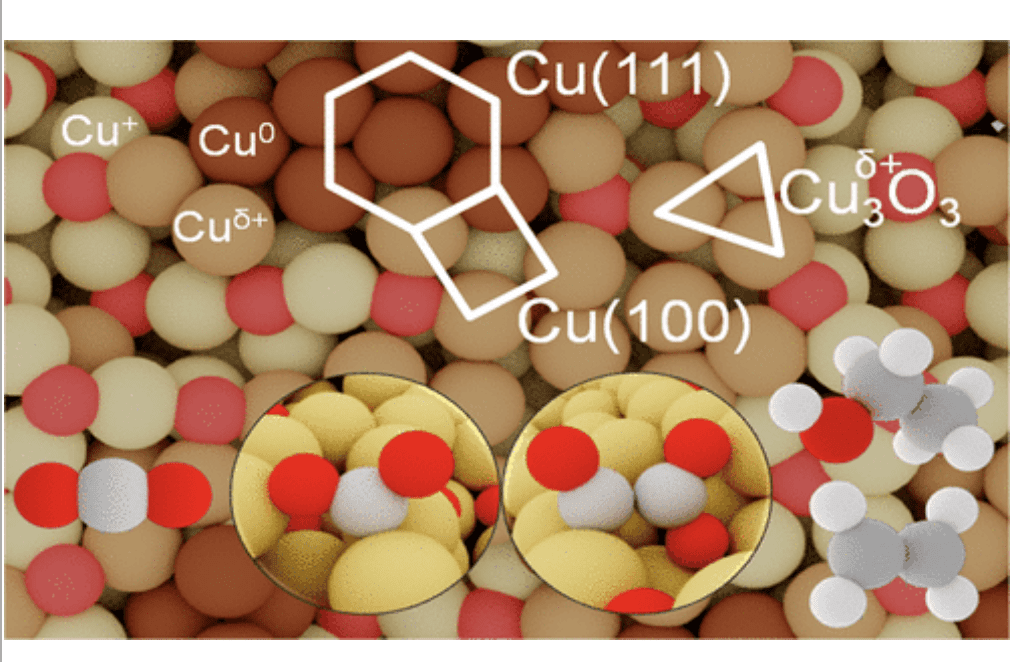
Copper catalysts are unique in CO2 reduction as they allow the formation of C2+ products. Depending on the catalysts’ synthesis, product distribution varies significantly: while Cu nanoparticles produce mainly methane and hydrogen, oxide-derived copper leads to ethylene and ethanol. Here, by means of ab initio molecular dynamics on oxygen-depleted models, we identified the ensembles controlling catalytic performance. Upon reconstruction and irrespective of the starting structure, recurrent patterns defined by their coordination and charges appear: metallic Cu0, polarized Cuδ+, and oxidic Cu+. These species combine to form 14 ensembles. Among them, 4-(6-)coordinated Cu adatoms and Cu3δ+O3 are responsible for tethering CO2, while metastable near-surface oxygens in fcc-(111) or (100)-like Cu domains promote C–C bond formation via glyoxylate species, thus triggering selective C2+ production at low onset potentials. Our work provides guidelines for modeling complex structural rearrangements under CO2 reduction conditions and devising new synthetic protocols toward an enhanced catalytic performance.
Dattila, F.; Garcı́a-Muelas, R.; López, N.
ACS Energy Lett. 2020, 5, 3176–3184
DOI:
10.1021/acsenergylett.0c01777

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















