10/04/2019
10/04/2019
 12:00 h
12:00 h
- Lecturer: Prof. Dr. Edwin Otten
- University: University of Groningen (The Netherlands)
- Sponsored by:

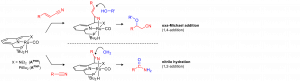
Nucleophilic (Conjugate) Addition to Nitriles via Metal-Ligand Cooperative Catalysis
Addition of nucleophiles to (unsaturated) nitriles presents significant challenges in comparison to other electron-withdrawing functional groups (aldehydes, esters etc.). The inherent low reactivity of nitriles is related to their polarization that is mostly due to inductive rather than resonance effects. Recently, we have established a nitrile-activation strategy that is based on metal-ligand cooperative binding of the CºN bond using Ru pincer complexes with a dearomatized pyridine backbone. The synergistic action of a Lewis basic ligand-site and a Lewis acidic metal centre results in a C=N fragment that shows significantly enhanced reactivity towards nucleophiles (see scheme). In this presentation, the application of this chemistry to challenging C-O bond formation will be described. Catalytic conjugate addition of alcohols to α,β-unsaturated nitriles (oxa-Michael addition), as well as direct nucleophilic addition of water to the CºN bond in aliphatic/(hetero)aromatic nitriles (hydration) will be highlighted, with a particular emphasis on the unusual metal-ligand cooperative mechanism of these reactions.
Other events

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements