Our research group is currently investigating transformations mediated by the reactivity of first row transition metals (1RTM). We are inspired by Nature, which employs 1RTM to carry out single and multi-proton/-electron transformations to sustain life. However, there are many fundamental questions with great technological potential which still remain unsolved. At present our attention is focused on carrying out i) multi-proton/-electron reactions such as water oxidation, water and CO2 reduction and reductions of organic compounds as well as ii) electro- and photocatalytic C-X (X = H, Cl or Br) activations mediated by biomimetic complexes based on earth abundant elements. From a conceptual point of view we seek to transfer concepts from artificial photosynthesis to the organic synthesis, to carry out endergonic transformations using sun-light as a driving force. We envision that these studies will trigger the development of new and greener methodologies for the transformation of organic molecules. Our approach implies the combination of photoredox and transition metal catalysis. To this end, we are developing and using high-throughput screening techniques (HST) to explore and expand these new methodologies. To obtain fundamental information about the operative mechanism, we use in-situ spectroscopic and spectrometric techniques, photo- and electrochemical studies, kinetic and labelling studies and theoretical modelling. Nevertheless, gaining insight into the understanding of the chemical reactivity is the primary aim of our research.
1. Multi Proton-Electron Transformations
Nature employs single and multi-electron redox transformations implemented in crucial metabolic routes that sustain life. Metalloenzymes are responsible forexecuting these challenging redox reactions in living organisms. Nature has found highly efficient active sites, bearing one or more redox active metals such as Mn, Fe, Ni, Co and Cu, which can carry out representative examples of these reactions such as light-driven water oxidation (CaMn4O4) and the final storage of sunlight into chemical bonds via CO2 reduction, H2 oxidation and proton reduction by hydrogenase enzymes (Fe and Ni), methane hydroxylation by soluble methane monooxygenase (sMMO) using O2 as the oxidant (Fe and Cu), the biological activity of vitamin B12 (Co), C-H oxidation by heme and non-heme enzymes (Fe) and dinitrogen fixation to ammonia (Fe, V, Mo). These are only some of the countless transformations which illustrate the great potential of those systems. However, our knowledge regarding the role of the metal center, the biological mechanisms and our capacity in mimicking their reactivity and selectivity is limited. The preparation and study of model systems to emulate this fascinating reactivity is linked to the basis of the chemical fundaments.
To emulate them, with synthetic model systems that perform efficient and selective transformations, we need to consider all aspects present in the metallozymes that enhance the reactivity towards the desired transformations. For instance, we need to manage a single proton and electron, in all versions of proton-couple-electron-transfer (PCET) processes. Likewise, we need to understand how to tune and control the metal ion oxidation states; the relationship between oxidation state, electron density, spin state and reactivity, and how the electronic and geometric modifications of the first coordination sphere and interactions of the second coordination sphere impact in the reactivity and selectivity. In order to gain some insight into these questions, we are working towards understanding the water oxidation, water reduction and CO2 reduction reactions by bio-inspired catalysts and the expansion to new organic transformations.
1.1 Water Oxidation
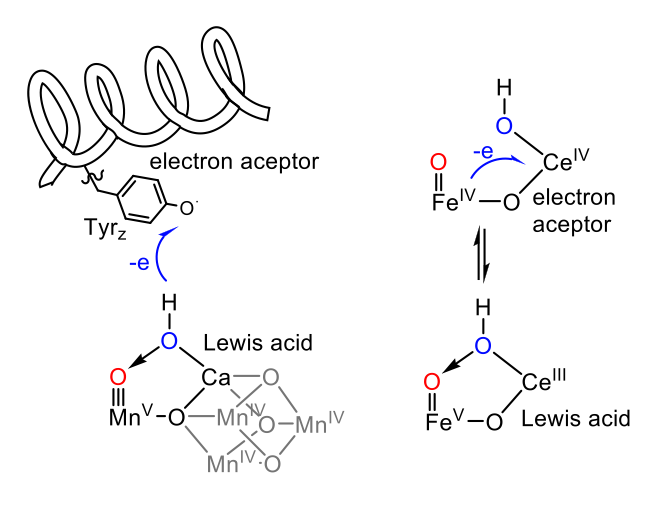
Water is the most appealing global-scale source of electrons that can be used to store energy into chemical bonds. However, the oxidation of the water molecule is an energetically uphill multi-proton-electron process, identified as one of the bottlenecks for the development of artificial photosynthesis. Water oxidation must to be catalysed in order to proceed at low energy barriers and practical reaction rates. Intermediates involved in water oxidation are highly unstable and their characterization is very challenging. Furthermore, despite the extensive efforts on the elucidation of the mechanism that operates in the oxygen evolving center (CaMnO4) located in the photosystem II (PSII), it is not yet fully understood. Several proposals are still under debate concerning the O-O formation mechanism and the role of calcium. Well-defined molecular complexes offer the possibility of studying the fundamental aspects of the water oxidation reaction, shedding light on the principles for a PSII understanding and efficient catalyst design.
On the other hand, catalysts based on earth-abundant metals are also particularly desirable because of their low cost and availability, iron being among the most attractive candidates. We have determined that iron complexes are highly efficient water oxidation catalysts.
Further studies of our well-defined water oxidation complexes have led us to identify a unique intermediate in water oxidation chemistry. We have spectroscopically characterized a FeIV-O-CeIV complex, which is the last intermediate prior to the rate determining step. Although similar intermediates have been previously proposed for ruthenium complexes, they have never been spectroscopically characterized.
On the other hand, the faster, most active and studied water oxidation catalysts are still based on ruthenium. Therefore, we examined and performed a direct comparison between homologous ruthenium and iron complexes water oxidation catalysts.
Our kinetics, spectroscopic, spectrometric and electrochemical studies, in combination with theoretical calculations, showed that both systems follow the same nucleophilic attack mechanism, where the reduction redox potential MV/IV dominates the catalytic activity.
2 (RuII-OH2) based on experimental and computational data.](https://www.iciq.org/wp-content/uploads/2014/11/Screen-Shot-2017-11-06-at-11.50.02.png)
Fig. 3. Proposed WO catalytic cycle for complex [RuII(OH2)(Py2Metacn)](PF6)2 (RuII-OH2) based on experimental and computational data.
These studies serve as an iron – ruthenium link and will aid in the future development of new and more effective WO catalysts.
1.2 Water and CO2 Reduction.
Metal complexes have proven to be very efficient in the catalytic water and CO2 reduction under electro- and photocatalytic conditions. However, the operating mechanisms are still not fully understood and efficiencies do not yet meet the requirements for industrial applications. Therefore, there is still a need for the development of new systems to extract mechanistic information, and the exploration of new concepts to enhance their reactivity and selectivity. Selectivity is indeed a key issue in CO2 reduction that can be controlled by molecular complexes. In addition, new reactivity could be envisioned from intermediates involved in these transformations. For example, metal complexes in low oxidation state and transition metal hydride complexes (M–H), are intermediates in hydrogen evolution catalysis. Therefore, new selectivities of reduction of organic molecules can be envisioned by following reaction pathways via M-H heterolytic (H− transfer) or homolytic (H• transfer) cleavage.
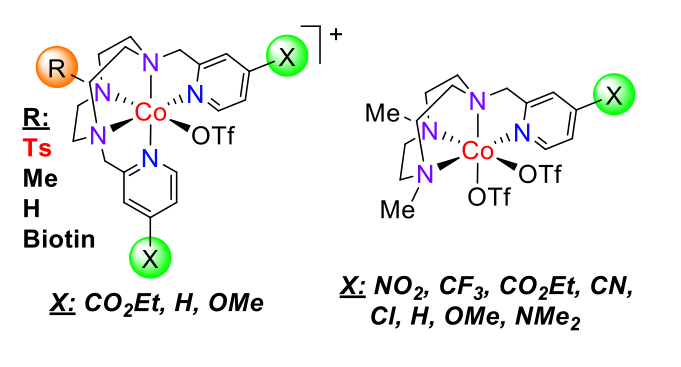
Fig.5. Cobalt aminopyridine complexes active in electro- and photocatalytic proton and CO2 reduction.
We are exploring new electro- and photochemical water reduction catalysts (WRC) [Co(Py2Tstacn)(OTf)](OTf) (Figure 5), to capture key intermediates in the photocatalytic water and CO2 reduction and characterize the protonation step by a combination of stoichiometric, kinetics, deuterium labelling, advanced spectroscopy and computational modelling.
We are also interested in metalloenzymes as a way to obtain more active and selective photo- and electrocatalyst catalysts.
.](https://www.iciq.org/wp-content/uploads/2014/11/Screen-Shot-2017-11-06-at-11.52.16.png)
Fig. 6 Line drawing structure of the developed cobalt complexes with the general formula [Co(XPy2Rtacn)(OTf)](OTf).
Mimicking natural photosynthesis has the potential to provide greener and light-driven methodologies for sustainable fuel production together with synthetic products. In both cases endergonic reactions must be carried out. Nevertheless, added value chemicals have less scaling and economic restrictions than the production of energy carriers. Therefore, the use of concepts derived from natural and artificial photosynthesis to perform selective transformations can provide the development of greener chemical transformations.
We are currently expanding this concept by employing designed cobalt complexes, based on aminopyridine ligands and initially developed for water reduction to hydrogen, in combination with a photoredox catalyst to reduce organic molecules.
We have developed a methodology for the light-driven reduction of ketones, aldehydes and olefins and the homocoupling of olefins. The reduction of these organic substrates in aqueous media is very remarkable since the putative metal-hydride intermediates are highly reactive towards protons. We have observed the appearance of new and surprising selectivities in this study. For instance, the high selectivity for the reduction of acetophenone versus aliphatic aldehydes. Indeed, this selectivity is unprecedented for previous metal catalyzed transformations. The benefits of the present system are avoiding the use of protecting-deprotecting steps and the use of stoichiometric amounts of lanthanides, which are required in other reduction methods of ketones and aldehydes.
2. Organometallic Reactivity.
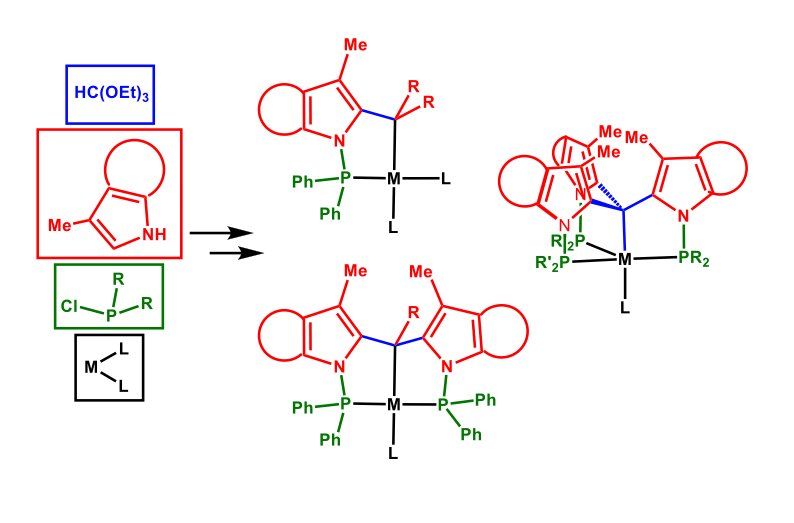
We are developing new organometallic complexes to activate small molecules. Our studies involve highly modular mono, bi and tridentate phosphine ligands with an accessible Csp3–H to produce P-C, P-C-P and CP3 metal complexes.
The readily available tris[(diphenylphosphino) -3-methyl-1H-indol-2yl] methane ligand (TPMI3CH) provides a highly rigid and well-defined coordination geometry, which has been successfully applied for the synthesis of C3-symmetric second and third row transition metal complexes where the apical Csp3–H is activated. Therefore, we decided to expand their reactivity to first row transition metal complexes, which is much more challenging, and study in detail the Csp3–H activation and Csp3–H···M interactions, which have an extraordinary relevance in inorganic, organometallic and synthetic chemistry. In this regard we have found that the Csp3–H is activated obtaining the nickel(II) halogenated complex. We are now exploring these highly robust organometallic complexes as potential catalysts for reduction of challenging substrates.
3. High-throughput experimental techniques.We efforts also include the development of devices to speed up catalytic studies. For example, we have developed parallel screening platforms of 24 and 48 reactions, enabling photocatalytic studies with temperature (-80 to 120 ºC) and light intensity control. Homogeneous irradiation in all reaction positions, allows us to also perform kinetic studies.
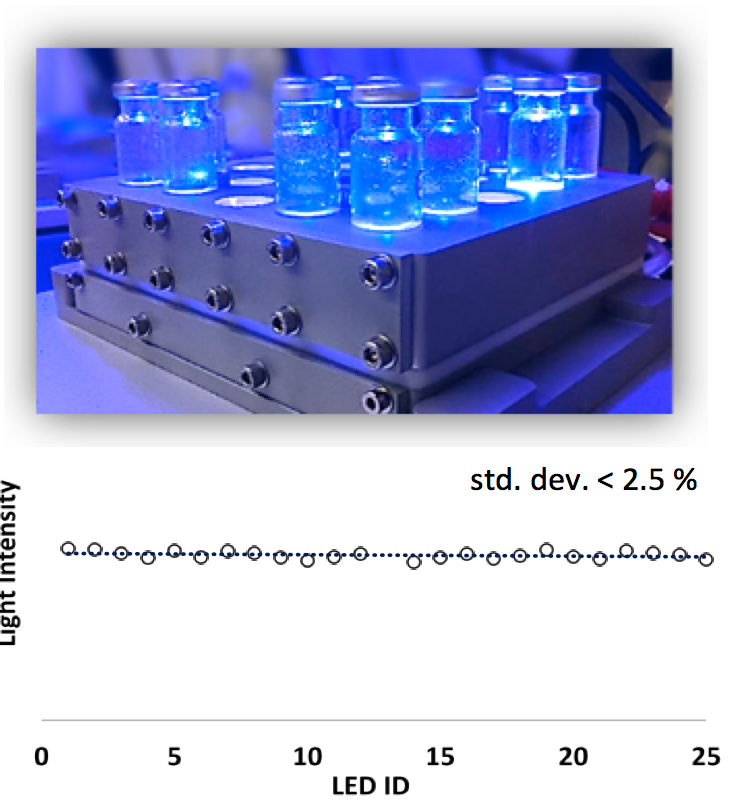
Fig 8. Top) High-throughput screening photoreactors (HTSP). Bottom) Comparison of the light intensity between all positions of the photoreactor at 700 mA



![Fig. 10 –[NiII(TPMI3C)Cl] metal complexes.](https://www.iciq.org/wp-content/uploads/2014/11/Screen-Shot-2017-11-06-at-11.54.33.png)