Our group combines the chemistry of small molecules and macromolecules to build functional materials. We are particularly interested in controlling the (mechanical) properties of the prepared materials by rational design. In our approach, chemistry is the thread that connects (macro)molecular structure, morphology, and function. Our efforts are aimed at developing strategies potentially applicable to sustainable development.
Polymer mechanochemistry
Polymer mechanochemistry studies the transduction of mechanical forces applied to polymeric materials from the macroscopic to the molecular scale. This effort is aided by so-called mechanophores, chemical motifs that generate physicochemical signals in response to structural changes induced by mechanical force. In many cases, the force-induced physicochemical signals produced by mechanophores are changes in the optical properties (absorption or luminescence) of materials caused by the cleavage of a covalent bond (weak bond) that is labile to force. Thus, mechanophores act as force sensors that allow one to read the mechanical history of a polymer. Understanding force-induced activation of mechanophores is an interesting fundamental research question that crosses several length scales and combines many disciplines. Solving this problem could create materials with better mechanical strength, for example.
Our group has developed a new class of mechanophores based on triarylmethanes (Tr). These species are known to generate stable carbocation (trityl, Tr+) by heterolytic cleavage, provided the aromatic rings are functionalized with electron-donor substituents to stabilize the positive charge. We have shown that the mechanical deformation of solid-state polymer materials can generate trityl cations. The force-induced heterolytic cleavage can be conveniently monitored since the starting triarylmethane (and polymers) are colorless, and the trityl carbocations absorb in the visible spectrum.
We are currently exploring several aspects of the mechanochemistry of triarylmethanes, including:
- Defining the chemical space (i.e., the combination of mechanophore and material design) within which the force-induced heterolytic cleavage can occur.
- Leverage the chemical nature of the mechanically generated species to trigger latent chemical functions (e.g., catalysis).
Polymer upcycling/recycling
The annual global production of virgin plastics is about 400 million metric tons. This impressive number highlights the central role that plastics have assumed in our daily lives. Still, it reminds us of the urgent environmental problem associated with their (uncontrolled) end-of-life scenarios. It is imperative to develop new polymers that possess properties that are competitive (or even better) than those of currently available commodity plastics and that are more prone to be recycled/degraded. Engineering this characteristic is particularly challenging, as the chemist has to find a trade-off between chemical degradability and stability under service conditions.
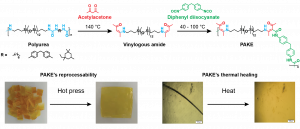
Our group has recently reported on the chemical upcycling of polyurea waste into new materials called polyaminoketoenamide (PAKE) networks. To achieve this goal, we developed a new chemical process consisting of the thermal degradation of urea bonds from the polyurea waste by treatment with acetylacetone, which produces vinylogous amide derivatives. These are further reacted with diisocyanates to obtain the PAKE networks. Thus, by replacing the urea bonds with aminoketoenamide bonds, we have shown that the polymer materials have become reprocessable, recyclable, and have far superior mechanical properties than those of the starting polyureas.
In another recent contribution, our group has shown that vinylogous urethane vitrimers, different polymer materials from the previously discussed PAKE, can be prepared by assembling small building blocks. These building blocks can be reobtained after the vinylogous urethane vitrimers are degraded. In other words, we have created a circular process in which the same feedstock is combined to create a plastic material and then properly recovered to reconstruct a brand-new material without resorting to a stream of new external chemicals, i.e., closed-loop recycling.