A novel methodology to produce reactive diazo compounds using highly available alkenes
9th maig 2024 -
The researchers from de group of Prof. Marcos G. Suero have asked themselves “Can we invent a disconnection approach to novel diazo compounds?”
The group of Prof. Marcos G. Suero (ICIQ) have described a new catalytic method to produce diazo compounds employing alkenes as starting materials based on a disconnection approach. This new diazo synthesis is unique among the classic synthetic routes. Moreover, this method allows access to β-alkoxydiazos, which is a class of novel diazo compounds highly reactive that have been unexplored due to the lack of synthetic methodologies.
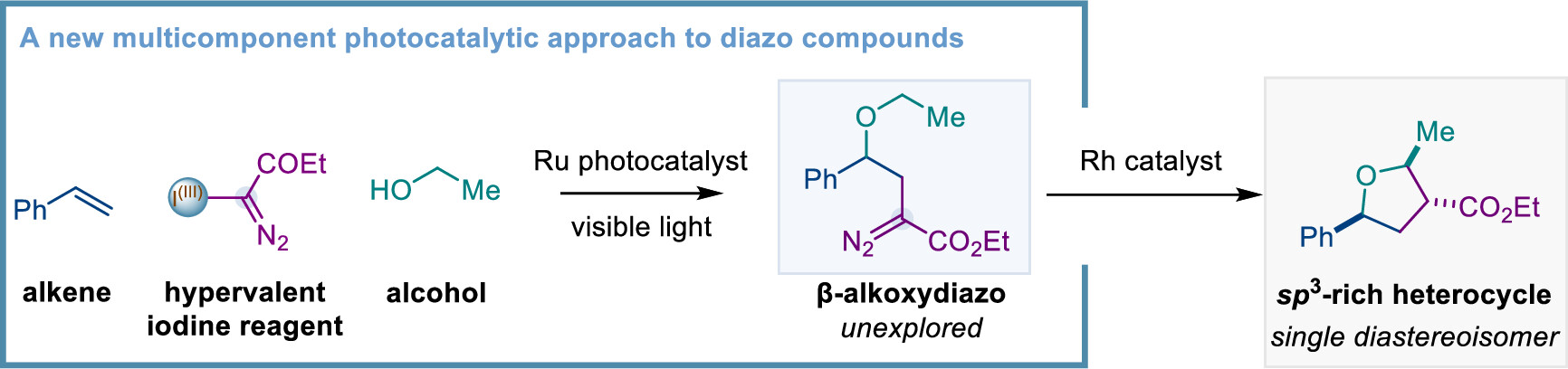
This work, published on the Journal of the American Chemical Society (JACS), present the discovery and development of the first photoredox-catalyzed alkoxy diazomethylation of alkenes with hypervalent iodine reagents and alcohols. This multicomponent process represents a new disconnection approach to diazo compounds and is featured by a broad scope, mild reaction conditions, and excellent selectivity.
“This work represents a new example of a formal carbyne transfer exploiting the dual radical−carbene reactivity of a hypervalent iodine reagent as a monovalent carbon precursor”
adds Quan Zhang, author of this work and PhD student at the group of Prof. G. Suero.
Diazo compounds have found wide and useful applications in synthetic organic chemistry, chemical biology and in the directed evolution of enzymes. Duo to the highly sensitive diazo functionality during the catalytic process, the catalytic synthesis using alkene starting materials has not been discovered. The successful development of this methodology represents a new disconnection approach using the most accessible building blocks as starting materials and that allows access to β-alkoxydiazos. Such type of diazo compounds represents an unexplored class which has not been made by other strategies, and the synthetic utility of such diazo compounds had been demonstrated in the construction of complex molecules via catalytic insertion reactions by metal-carbene species.
Notícies relacionades

Creem un futur més brillant
Uneix-te al nostre equip per treballar amb investigadors reconeguts i fer front a les novetats
projectes i contribuir a avenços científics significatius







 27-03-2025
27-03-2025