Semàfor químic
Objectiu: Canviar el color d’una solució només sacsejant-la

-
Materials de laboratori
1 Erlenmeyer
3 espàtules
1 ampolla de vidre amb una tassa
-
Reactius
Hidròxid de sodi (NaOH)
Glucosa
Indigo Carmine
Aigua
-
Seguretat
No oblideu els guants, la bata de laboratori i les ulleres de seguretat!!!
-
Preguntes
Quin tipus de reacció està tenint lloc?
D'on surten tots els colors?
Procediment
- Dins d’un matràs Erlenmeyer, afegiu 100 ml d’aigua destil·lada.
- Pesar i afegir 1,5 g de NaOH i remenar fins que es dissolgui.
- Pesar i afegir 2 g de glucosa i remenar fins que es dissolguin.
- Afegiu una petita quantitat d’índigo carmí i barregeu bé.
- Espereu fins que la solució es torni groga, remeneu-la i observeu què passa. El procés pot trigar uns minuts.
Explicació teòrica
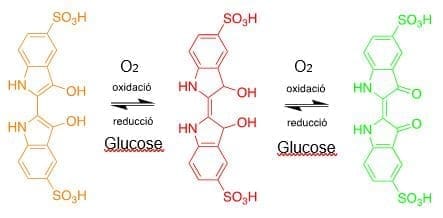
Els colors observats en aquest experiment sempre provenen del carmí indigo. Quan s’agita la mescla, entra l’oxigen de l’aire i s’oxida primer l’índigo carmí fins a la seva forma vermella, i si seguim agitant, podem veure la forma verda. Quan deixa de remenar, l’oxigen es consumeix, i l’indigo carmí reacciona amb la glucosa present reduint-la a la forma vermella, i finalment torna a groc. L’hidròxid de sodi s’utilitza com a base per facilitar totes aquestes reaccions redox.
Aquí teniu un esquema de les reaccions redox amb els diferents colors de l’indi carmí en medi bàsic:

Creem un futur més brillant
Uneix-te al nostre equip per treballar amb investigadors reconeguts i fer front a les novetats
projectes i contribuir a avenços científics significatius














