2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004
-
New Tools for Taming Complex Reaction Networks: The Unimolecular Decomposition of Indole Revisited
GO TO OPEN ACCESS ACS Phys. Chem Au 2022, 2 (3), 225–236, DOI: 10.1021/acsphyschemau.1c00051.
-
Grafting of Anionic Decahydro-Closo-Decaborate Clusters on Keggin and Dawson-Type Polyoxometalates: Syntheses, Studies in Solution, DFT Calculations and Electrochemical Properties
GO TO OPEN ACCESS Molecules 2022, 27 (22), 7663, DOI: 10.3390/molecules27227663. -
Computational Prediction of Speciation Diagrams and Nucleation Mechanisms: Molecular Vanadium, Niobium, and Tantalum Oxide Nanoclusters in Solution
Inorg. Chem. 2022, 61 (35), 13708–13718, DOI: 10.1021/acs.inorgchem.2c00925.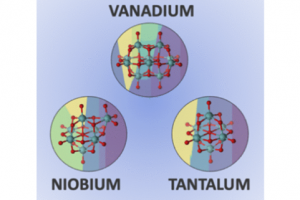
-
Mechanistic insights of molecular metal polyselenides for catalytic hydrogen generation
GO TO OPEN ACCESS Chem. Commun. 2022, 58, 6906-6909, DOI: 10.1039/D2CC01226J.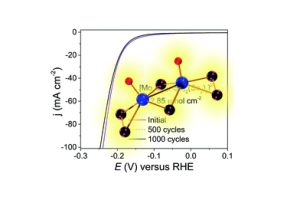
-
Chemical reaction network knowledge graphs: the OntoRXN ontology
J. Cheminformatics 2022, 14, 29, DOI: 10.1186/s13321-022-00610-x.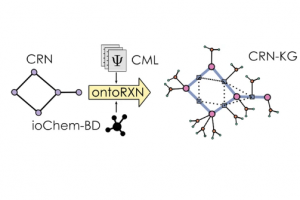
-
Unparalleled selectivity and electronic structure of heterometallic [LnLn′Ln] molecules as 3-qubit quantum gates
GO TO OPEN ACCESS Chem. Sci. 2022, DOI: 10.1039/D2SC00436D.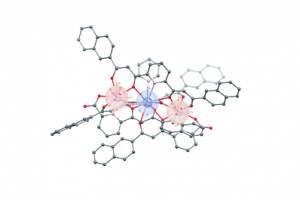
-
Domino Synthesis of Bicyclic 3,5-Anhydro Furanose Mimics Using a Binary Al(III) Complex/Halide Catalyst
ACS Catal. 2022, 12, 5464–5469, DOI: 10.1021/acscatal.2c00925.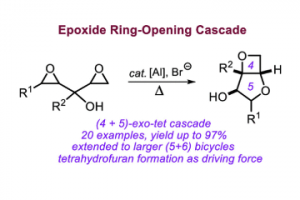
-
Elucidating Sulfide Activation Mode in Metal-Catalyzed Sulfoxidation Reactivity
GO TO OPEN ACCESS Inorg. Chem. 2022, DOI: 10.1021/acs.inorgchem.2c00037.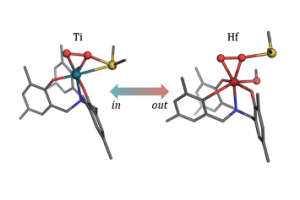
-
Predicting the Solubility of Inorganic Ions Pairs in Water
Angew. Chem. Int. Ed. 2022, e202117839, DOI: 10.1002/anie.202117839.
-
Cascade Transformation of Carbon Dioxide and Alkyne-1,n-diols into Densely Substituted Cyclic Carbonates
GO TO OPEN ACCESS ACS Catal. 2022, 12, 2854–2860, DOI: 10.1021/acscatal.1c05773.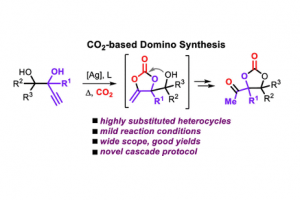
-
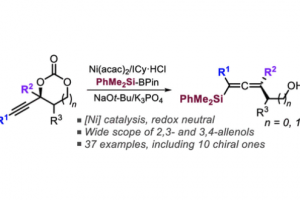
Ni-Catalyzed Decarboxylative Silylation of Alkynyl Carbonates: Access to Chiral Allenes via Enantiospecific Conversions
Org. Lett. 2022, 24 (2), 637–641, DOI: 10.1021/acs.orglett.1c04086.