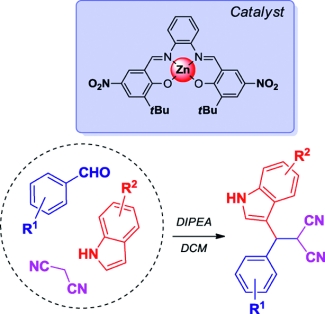
The synthesis of 3-substituted indoles was investigated through a multicomponent reaction (MCR) approach by using aldehydes, indole and malononitrile as the reagents. The reaction was catalyzed by Lewis acidic Zn(salphen) complexes and their performance was compared with a number of other ZnII structures and M(salphen)s, which showed the Zn(salphen)s to be superior. However, the complex nature of this three-component reaction (3-CR) results in substantial byproduct formation that arises from the intermediate benzylidene malononitrile species. The 3-CR was studied in detail that covered the influence of the base, solvent, reagent stoichiometry and also involved stability studies. The results led to a mechanistic proposal in which the benzylidene malononitrile intermediate plays a central role; it is one of the major species that is formed in most of the catalytic reactions studied. Furthermore, it provided a prelude for the in situ reaction with the malononitrile reagent, which most probably affords a complex mixture of N-containing heterocycles.