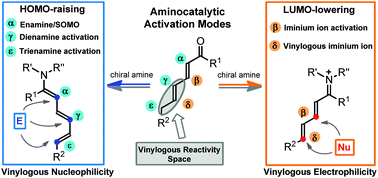
Over the past decade, asymmetric aminocatalysis has become a reliable synthetic platform for generating stereogenic centres at the α and β positions of unmodified carbonyl compounds with very high fidelity. More recently, chemists have become interested in using aminocatalysis for targeting stereocentres even more remote from the catalyst’s point of action. The key to success is the ability of the amine catalyst to propagate the electronic effects inherent to aminocatalytic reactivity modes (i.e. the HOMO-raising and the LUMO-lowering activating effects) through the conjugated π-system of poly-unsaturated carbonyls while transmitting the stereochemical information at distant positions. This feature article outlines how the combination of aminocatalysis with the principle of vinylogy has brought about the development of dienamine, trienamine, and vinylogous iminium ion activations, novel strategies for the asymmetric functionalisation of carbonyl compounds at their γ-, ε-, and δ-positions, respectively.