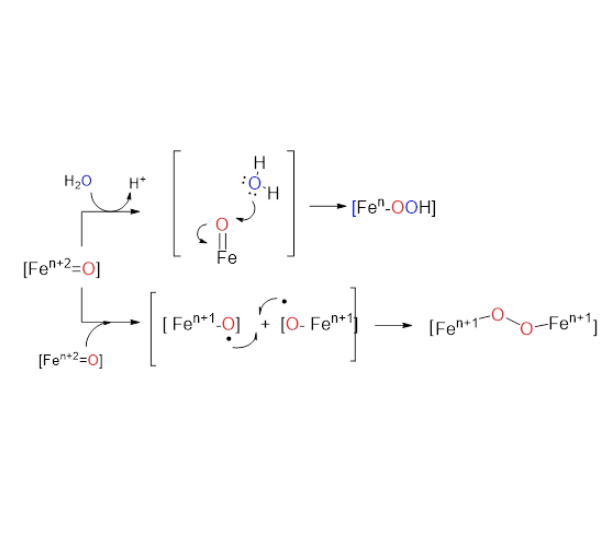
Iron is an exceptional chemical element due to its low cost, abundance and biocompatibility, but also in terms of the accessible redox states. As a natural evolution to the rich oxidative chemistry that can mediate water oxidation catalysts, during the last decade, iron coordination complexeshave emerged as a promising platform for investigation. In this chapter the main progress on the design of well-defined water oxidation (WO) catalysts based on iron is covered, as well as evidence for the WO proposed mechanisms. Ligand design is critical for the rationalization of the catalytic activity and therefore the different architectures are used as the crucial pillar structure of the chapter. Over the different sections, we present detailed characterization of reaction intermediates and provide insights into our understanding of the O–O bond formation event. In this regard, the most illustrative studies are those based on chemical oxidants. Electrocatalysis and electrode functionalization studies, although still very preliminary, indicate that possibilities toward applications could materialize. The few studies of the challenging light-driven water oxidation are also covered. Finally, multinuclear iron complexes are of particular interest, since they provide a clear route for the development of electrocatalysts. In addition, a discussion of metal organic frameworks(MOFs) and polyoxometalates (POMs) is also included in this chapter.