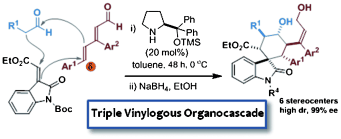
We report a triple vinylogous cascade reaction, yielding valuable spiro-oxindolic cyclohexane derivatives. The three-component domino process proceeds by way of a catalyzed Michael/1,6-addition/vinylogous aldol sequence affording the products with six stereogenic centers and very high control over the stereochemistry. The chemistry is based on a rare example of asymmetric 1,6-addition to linear 2,4-dienals proceeding with complete δ-site selectivity. Key to the reaction development was a directing group positioned at the β-dienal position, which was essential for achieving highly predictable.