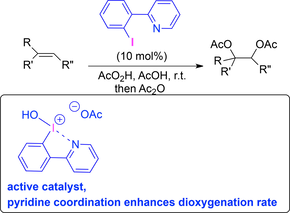
The influence of a 2-pyridinyl substituent on the catalytic performance of aryl iodides as catalyst in iodine(III) chemistry was explored. An efficient Lewis base adduct between the pyridine nitrogen and the electrophilic iodine(III) center was identified and confirmed by X-ray analysis. This arrangement was shown to generate a kinetically competent superior catalyst structure for the catalytic dioxygenation of alkenes. It introduces the concept of Lewis base adduct formation as a kinetic factor in iodine(I/III) catalysis.