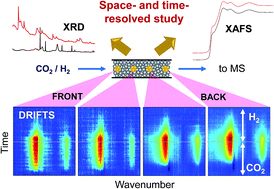
CO2 capture-reduction (CCR) is a recently developed catalytic process that combines two critical functions of the CO2 utilization path in one process, namely CO2 capture and subsequent transformation (e.g. reduction by H2) into chemical fuels or intermediates such as CO. A bifunctional catalyst material is needed and the two functions are activated by means of an isothermal unsteady-state operation (i.e. gas switching). This work employs operando space- and time-resolved DRIFTS, XAFS, and XRD to elucidate the nature and functions of Cu and the promoters. Both unpromoted and K/Ba-promoted Cu/Al2O3 catalysts were studied to illuminate the active surface species varying along the catalyst bed. The K promoter was found to uniquely facilitate efficient CO2 capture in the form of surface formates, dispersion of active metallic Cu and suppression of surface Cu oxidation. The CO2-trapping efficiency of the K-promoted catalyst is so high that CO2 capture takes place gradually along the catalyst bed towards the reactor outlet, hence creating large spatial and temporal gradients of surface chemical species. Understanding these features is of central importance to design efficient CCR catalysts. Furthermore, a completely different path for CO2 reduction was evidenced for the unpromoted and Ba-promoted Cu catalysts where CO2 can directly react with metallic Cu and oxidize its outer surface and thus releasing CO. These results also provide important new mechanistic insights into the widely investigated reverse water–gas shift reaction and the role that K and Ba promoters play.