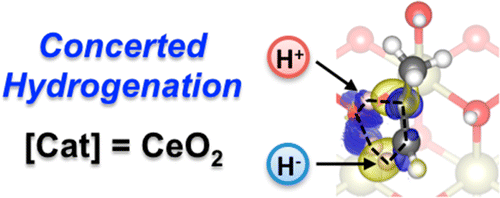
Despite its ubiquity in homogeneous and enzymatic catalysis, concerted mechanisms have been overlooked for heterogeneously catalyzed reactions. The elusive nature of transition states leaves Density Functional Theory, DFT, as the only robust tool for their identification and characterization. By means of this method, we show that a concerted path takes part in the recently discovered semihydrogenation of propyne on CeO2, for which an excellent activity and selectivity have been reported. The high surface H coverage imposed by the experimental hydrogenation conditions induces site isolation and drives the reaction through a six-membered ring transition state. This unprecedented pathway accounts for many of the experimental observations, such as the unique syn-stereoselectivity, the excellent alkene selectivities, or the high temperature and large H2/alkyne ratios required.