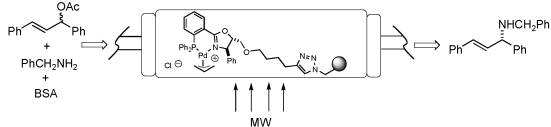
A family of enantiopure diphenylphosphinooxazolines (PHOX) containing in their structures a sterically tunable alkoxymethyl group (-CH2OR) has been optimized for the palladium-catalyzed asymmetric allylic amination. The optimal catalyst (R=CH3), depicting very high catalytic activity and broad scope applicability, has been further modified to include an ω-alkynyloxy substituent of variable length for polymer supporting via click chemistry, and has been anchored onto slightly cross-linked azidomethyl poly(styrene). The length of a polymethylene chain connecting the PHOX unit with the 1,2,3-triazole linker has been optimized, and the first polymer-supported PHOX ligands for the highly enantioselective allylic amination have been prepared in this manner. Conditions for catalyst recovery and reuse in microwave-promoted amination reactions have been established, and the system has been finally adapted to continuous flow operation.